What is pH measurement?
As wide variety of applications in industries because in industries there is a various solutions used so we want to find out whether the solutions are in acidic in nature or alkalinity in nature. It is essential because when we know about the acidity or alkalinity of the solution in chemical process can be proceed effectively. So it is very necessary to find out the pH of the solutions.
pH measurement is the wide variety of industrial applications mainly for water and waste water treatments.
To find out the alkalinity and acidity of the solutions. pH is a measure of acidity or alkalinity of the aqueous solution. Aqueous solutions is diluted and dissolved in water which are water in their solvent.
Acids are when react with water acids it is produces the hydrogen ions, bases are those dissolved with water and produces the hydroxyl ions.
Acids = HA = H⁺ + A⁻
HA- acid, H⁺ – hydrogen ion, A⁻ – anion
Base = MOH = M⁺ + OH⁻
MOH – Base, M⁺ – cation, OH⁻ – hydroxyl ion
To find out the solutions whether it is acidic or base in nature,
If the hydrogen ion is more it is acidic in nature, if hydroxyl ion concentration is more it is base in nature.
To measure the pH in solutions the hydrogen ions and hydroxyl ion concentration is to be measured.
The concentration of H⁺ and OH⁻ is equal to dissociation constant.
(H⁺) × (OH⁻) = Kw= 10⁻ 14 (at 25 degree)
The pH solution is defined as the negative logarithmic of the hydrogen ion concentration
pH= -log 10(H⁺)
the hydrogen ion concentration is measured on the scale which is known as the pH scale. The scale is the range of 0 to 14.
Let us how pH can be calculated. In the solution we are having the hydrogen concentration as
10⁻14 = [H⁺]
pH = -log 10 [10⁻14]
pH= 14
pH scale is divided into three parts
- Acidic
- Neutral
- Base
Acidic
The acidic is in the range of 0 to less than 7
Neutral
The pH value is 7 constant
Base
The pH value is greater than 7
When the solution is a start increasing from 0 to 14 then the solution is became the base, when its starting decreasing solution becomes acidic in nature.
There are various methods are used for pH measurement, let us see the electrical method
In electrical method a pair of electrodes is immersed in the solution this pair of electrodes they are having two electrodes one electrode is measuring electrode and other is reference electrode, with these pairs of liquids are pH is measured.
Construction
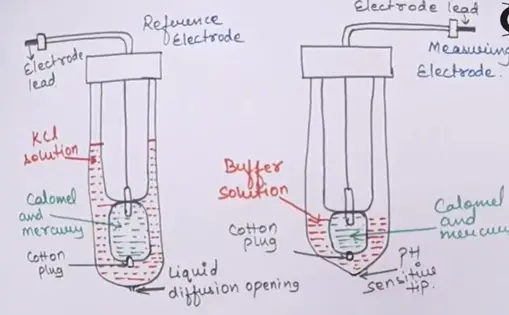
There are two types of electrodes
- Measuring electrodes
- Reference electrodes
- These electrodes are made up of glass tubes
- In glass tube having inner assembly ,in this inner assembly filled with calomel and mercury in both measuring and reference electrode.
- In the electrodes lead is inserted into the mercury.
- There is a cotton plug is to close bottom hole of the electrode where the mercury is not be mixed with the outer solutions.
- In outer side KCL solution is present in reference electrode and buffer solution is present in the measuring electrode.
- Kcl solution has a constant pH.
- In bottom of there is a tiny hole is presented in reference electrode is called the liquid diffusion opening is to empty the electrode.
- In measuring electrode the bottom tip is closed this tip is acts as the pH sensitive tip.
- When this tip is contact with the solution or immersed in solution the pH is measured.
Working
The reference electrode is in electrical contact with the solution whose pH is to be measured.
When the measuring electrode is immersed in the measuring solution, when the buffer solution and measuring solution contact at that point the voltage is generated. That voltage is measured with the help of voltmeter or the pH calibrated controller.
In the reference electrode the KCL solution has a constant pH is measured.
Another method is limitus paper method (strips)
It gives two stages Acid and base.
Acid changes the colour from blue to red
Base changes from red to blue
Advantages
- Simple to use
- Calibration control
- Portable and cheap
Disadvantage
- The glass tube to be cleaned regularly
- It is corressive.