HOW LITHIUM- ION BATTERY WORKS
The portable power supply is become the lifeline in the modern technological world especially lithium ion battery.
Here we are going to discuss how lithium ion battery works by using Tesla principle.
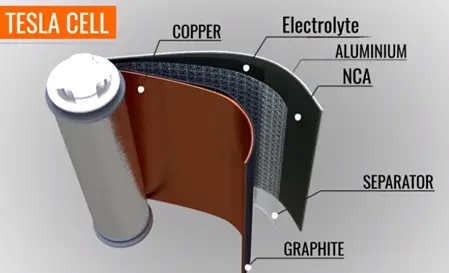
Lithium ion battery has different layers inside. Tesla lithium ion battery works on intensity concept associated with metals called electrochemical potential.
Electrochemical potential is tendency of metal to loose electrons. In fact very first cell developed by Alessandro Volta more than 200 years ago based on the concept of electro chemical potential.
Electro chemical series
Li : 3.04V
Mg : 2.37V
Al : 1.66V
Zn : 0.76V
Fe : 0.44V
H : 0 V
Hg : -0.24 V
Cu : – 0.34 V
Ag : -1.69 V
F : – 2.8 V
According to this lithium has the tendency to lose more electrons. Florine is least tendency to loose electrons.
Volta took two metals with differential chemical potential zinc and silver created an external flow of electricity.
Sony made the first commercial model lithium ion battery in 1991. It’s based on the same concept of electro chemical potential. Lithium has a highest tendency to loose electrons was used in lithium ion cells. Lithium has only one electron in outer shell and always wants to loose these electrons due to this reason pure lithium is highly reactive metal even it reacts with water and air.
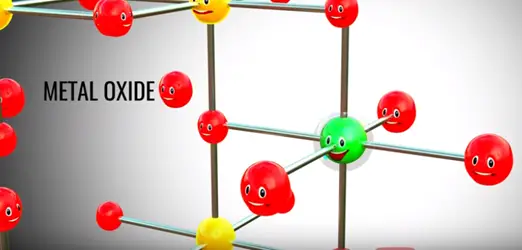
The trick lithium ion battery operation is that fact lithium in its pure form ite reactive metal. When the lithium is part of metal oxide it is quite stable.
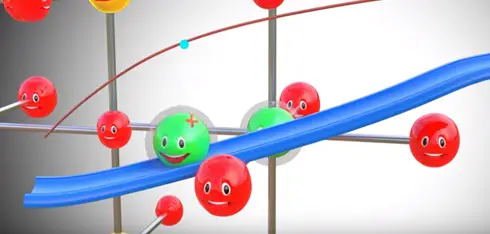
Assume somehow we have separated lithium atom from this metal oxide lithium atom is highly unstable for lithium ion and electrons is however lithium is a part of metal oxide is much more stable in this state. If we provide two different paths for electrons and lithium ion flows between the lithium and metal oxide lithium atom automatically reach metal oxide.
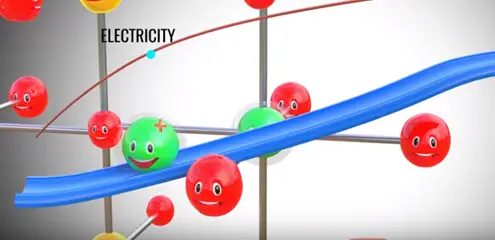
During this process we have produced electricity electron flow through one path by this process electricity is produced in lithium metal oxide.
Let us see how lithium ion cells achieve these two objectives.
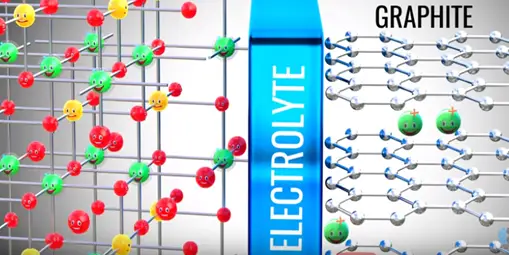
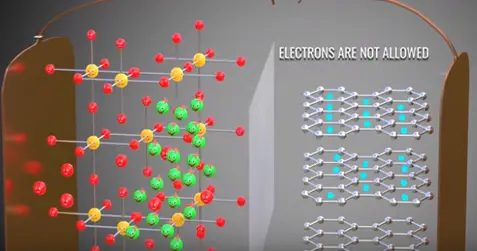
A practical lithium ion cell uses an electrolyte and graphite. Graphite has a layered structure these layers are loosely bonded so that separated lithium ions can be stored easily.
In between the electrolyte the graphite and metal oxide access as guard which allows lithium ion pass through it.
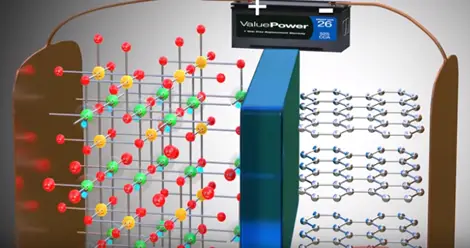
Let us see when power source is connected in this arrangement.
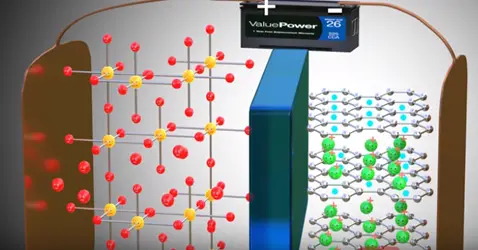
The positive side of the power source is attracted and removed electrons from the lithium atoms of metal oxide. These electrons flows through the external circuit cannot flow through the electrolyte and reach the graphite layer. In mean time positive charged lithium ion will be attracted towards the negative terminal and flow through the electrolyte. Lithium ion also reaches the graphite layer space and trapped there. Once lithium ion reaches graphite sheet cell is fully charged this is we have achieved first objective. The lithium ion and electrons detached from the metal oxide.
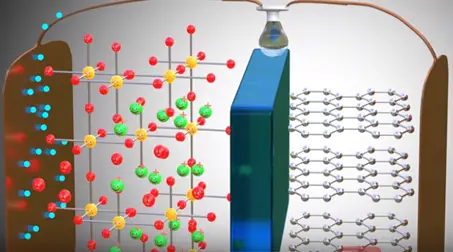
As soon as power source is removed and load is connected.
Lithium ion wants to go back to its stable state it is part of metal oxide due to this tendency lithium ions move through the electrolyte the electrons via the load. Thus we get an electrical current through the load. The graphite has role in the chemical reaction in lithium ion cells.
The graphite is just storage medium for lithium ions. If the internal temperature is rises due to abnormal condition liquid electrolyte will dry up and there will be short circuit between the anode and cathode this can be fire or exposure. To avoid this situation the insulating layer called separator is placed between the electrodes separator is permeable for lithium ions because it is microprocessor.
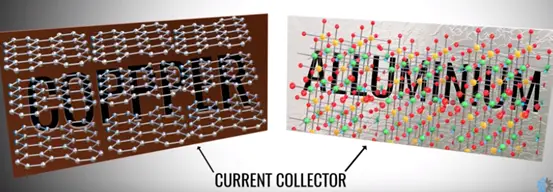
In a practical cell the graphite and metal oxide coded with copper and aluminium foils. The foils act as a current collector.
The positive and negative tabs take over easily from collectors. The electrolyte is coded on the separator sheet all these three sheets round on the cylinder center sheet core that makes the compact.
The standard Tesla cell has a standard voltage between 3- 4.2 volts. Many Tesla cells connected in series in parallel fashion to form majo. If such majo are connected in series to form a battery pack in a car.
Lithium ions cells produced more heat during operation high temperature decade the cells performance. The battery management system used to manage the temperature state change of voltage protection and self-monitoring such as huge number of cells. Glycol based cooling technology is used to test the battery pack. BMS is the glycol flow rate to maintain the optimum battery temperature.
While the cells charging higher capacity cells is charged faster to solve this problem. BMS used method is called cell balancing.
In cell balancing all the battery allowed to charge cells equally that protecting them from the over voltage.
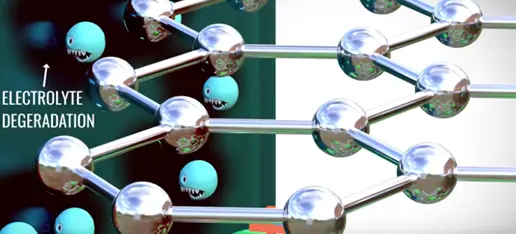
There is a magical phenomenon which happens in lithium ion cells during their very first charge that saves the lithium cells sudden death. Electron graphite layer are major problem the electrolyte is degraded if the electrons contact with it.
However electrons never contact with electrolyte due to accidental discovery the solid electrolyte interface (SEI).
When you charge first time lithium ion moves through the electrolyte. In this journey solvent molecule covers the lithium ions. When they reach the graphite the lithium ions along with the solvent molecules react with graphite and form a layer called SEI layer. The formation of SEI layer is blocking and prevents any direct contact between electrons and electrolyte thus saving electrolyte from dehydration. This over all formation of SEI layer consumes 5% of lithium the remaining 95% of lithium contributes main working of battery.