SI Units System
The International System of Units (SI, abbreviated from the French Système international (d’unités)) is the modern form of the metric system, and is the most widely used system of measurement. It comprises a coherent system of units of measurement built on seven base units (ampere, kelvin, second, metre, kilogram, candela, mole) and a set of twenty decimal prefixes to the unit names and unit symbols that may be used when specifying multiples and fractions of the units. The system also specifies names for 22 derived units for other common physical quantities like lumen, watt, etc.
Base units
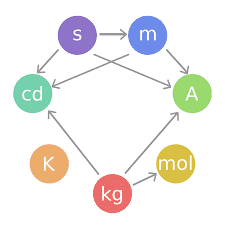
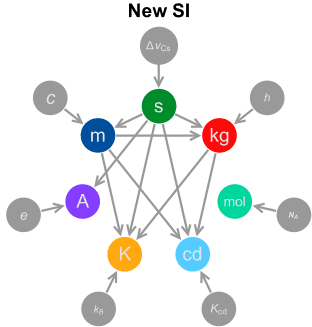
The SI base units are the building blocks of the system and all the other units are derived from them. When Maxwell first introduced the concept of a coherent system, he identified three quantities that could be used as base units: mass, length and time. Giorgi later identified the need for an electrical base unit, for which the unit of electric current was chosen for SI. Another three base units (for temperature, amount of substance and luminous intensity) were added later.
[wp_ad_camp_1]
| SI base units | ||||
| Unit name |
Unit symbol |
Dimension symbol |
Quantity name |
Definition |
| metre | m | L | length |
|
| kilogram | kg | M | mass |
|
| second | s | T | time |
|
| ampere | A | I | electric current |
|
| kelvin | K | Θ | thermodynamic temperature |
|
| mole | mol | N | amount of substance |
|
| candela | cd | J | luminous intensity |
Note: both old and new definitions are approximately the luminous intensity of a whale blubber candle burning modestly bright, in the late 19th century called a “candlepower” or a “candle”. |
Notes
- Interim definitions are given here only when there has been a significant difference in the definition.
- Despite the prefix “kilo-“, the kilogram is the base unit of mass. The kilogram, not the gram, is the coherent unit and is used in the definitions of derived units. Nonetheless, prefixes are determined as if the gram were the base unit of mass.
- In 1954 the unit of thermodynamic temperature was known as the “degree Kelvin” (symbol °K; “Kelvin” spelt with an upper-case “K”). It was renamed the “kelvin” (symbol “K”; “kelvin” spelt with a lower case “k”) in 1967.
- When the mole is used, the elementary entities must be specified and may be atoms, molecules, ions, electrons, other particles, or specified groups of such particles.
The Prior definitions of the various base units in the above table were made by the following authorities:
- FG = French Government
- IEC = International Electro Technical Commission
- ICAW = International Committee on Atomic Weights
All other definitions result from resolutions by either CGPM or the CIPM and are cataloged in the SI Brochure.
New SI units System:
Derived units
The derived units in the SI are formed by powers, products or quotients of the base units and are unlimited in number. Derived units are associated with derived quantities; for example, velocity is a quantity that is derived from the base quantities of time and length, and thus the SI derived unit is metre per second (symbol m/s). The dimensions of derived units can be expressed in terms of the dimensions of the base units.
[wp_ad_camp_1]
Combinations of base and derived units may be used to express other derived units. For example, the SI unit of force is the newton (N), the SI unit of pressure is the pascal (Pa)—and the pascal can be defined as one newton per square metre (N/m2).
| Named SI derived units | ||||
| Namenote 1 | Symbol | Quantity | In other SI units | In SI base units |
| radiannote 2 | rad | angle | (m⋅m−1) | |
| steradiannote 2 | sr | solid angle | (m2⋅m−2) | |
| hertz | Hz | frequency | s−1 | |
| newton | N | force, weight | kg⋅m⋅s−2 | |
| pascal | Pa | pressure, stress | N/m2 | kg⋅m−1⋅s−2 |
| joule | J | energy, work, heat | N⋅m = Pa⋅m3 | kg⋅m2⋅s−2 |
| watt | W | power, radiant flux | J/s | kg⋅m2⋅s−3 |
| coulomb | C | electric charge or quantity of electricity | s⋅A | |
| volt | V | voltage (electrical potential), emf | W/A | kg⋅m2⋅s−3⋅A−1 |
| farad | F | capacitance | C/V | kg−1⋅m−2⋅s4⋅A2 |
| ohm | Ω | resistance, impedance, reactance | V/A | kg⋅m2⋅s−3⋅A−2 |
| siemens | S | electrical conductance | Ω−1 | kg−1⋅m−2⋅s3⋅A2 |
| weber | Wb | magnetic flux | V⋅s | kg⋅m2⋅s−2⋅A−1 |
| tesla | T | magnetic flux density | Wb/m2 | kg⋅s−2⋅A−1 |
| henry | H | inductance | Wb/A | kg⋅m2⋅s−2⋅A−2 |
| degree Celsius | °C | temperature relative to 273.15 K | K | |
| lumen | lm | luminous flux | cd⋅sr | cd |
| lux | lx | illuminance | lm/m2 | m−2⋅cd |
| becquerel | Bq | radioactivity (decays per unit time) | s−1 | |
| gray | Gy | absorbed dose (of ionizing radiation) | J/kg | m2⋅s−2 |
| sievert | Sv | equivalent dose (of ionizing radiation) | J/kg | m2⋅s−2 |
| katal | kat | catalytic activity | mol⋅s−1 | |
Ref: https://en.wikipedia.org/wiki/International_System_of_Units
License: Creative Common share a like











![What is Arc Chute? Types, Working Principle [Video Included] arc chute working priciple](https://electrical4u.net/wp-content/uploads/2020/06/arc-chute-218x150.png)