Zinc Phosphate Formula
Zinc phosphate is an inorganic compound that has contains the crystalline structure. It has more toxicity substances when it reacts with lead or chromium. This is applied as the primer pigment. The seeding agent is often used as a pre-treatment. The commont agent that can be used is sodium pyrophosphate.The systematic IUPAC name is known as zinc phosphate. The chemical or molecular formula of zinc phosphate is Zn3(PO4)2.
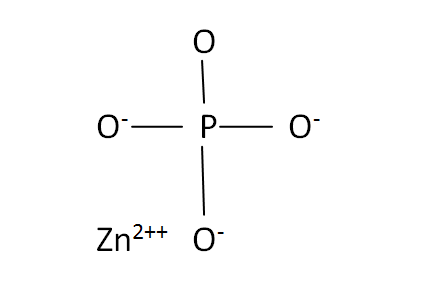
Structural Formula
This is the structural formula of the zinc phosphate:
Chemical Formula
The chemical formula of the zinc phosphate is Zn3(PO4)2.
Preparation
It can be produced by the presence of the phosphate rich solutions. When the magnesium oxide and zinc oxide powders consists the liquids such as water,phosphoric acid and buffers. It has increasingly the adhesion properties of the paint on the wall. The different forms of the zinc orthophosphate and the mineral has consists of the tarbutitte.
Physical Properties
| Melting point | 900C |
| Boiling point | 158C |
| Molecular weight | 386.11g/mol |
| Density | 3.998g/cm3 |
| Solubility in water | Insoluble |
| Refractive index | 1.595 |
| Appearance | White solid |
| Crystal structure | monoclinic |
Chemical Properties
It has more toxic to the animals such as rabbits, rats and mice. This is given to the primary coating that interacts between the external agent and internal agent. When it is not used the different temperature using through the latex tube. It is mostly occur in the earth’s crust and should be easily immersed.
Uses
It is used to fill the damaged teeth in the industry. It is used as a plating agent and surface treating agent. It is used to anti scaling properties. For the production of rubber products and anti scaling agent it is used. Dyes is prepared from the zinc phosphate. It is used to preserve from wall paint additive. In industry it is used to corrosive inhibitor. It is also used for water treatment products.