Zinc Nitrate
Zinc nitrate is an inorganic compound that is typically encountered at highly deliquescent substances. It is soluble both in the water and alcohol. It is soluble in water and alcohol. It is formed by the zinc is mixed with the nitric acid. It is found abundantly in the hexahydrate. It has a colourless and crystalline solid. While it is considered as the zinc is cations and nitrate is anions. The systematic IUPAC name is known as zinc nitrate. The chemical or molecular formula of zinc nitrate is Zn(NO3)2. It is also known as zinc dinitrate.
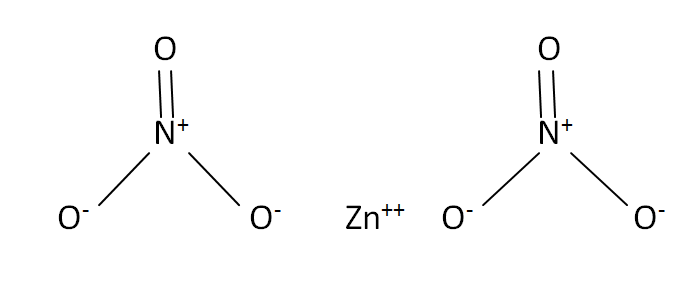
Structural Formula
This is the structural formula of the zinc nitrate:
Chemical Formula
The chemical formula of the zinc nitrate is Zn(NO3)2.
Preparation Method
It can be prepared by the reacting with nitric acid because it the highly corrosive minerals acid. Based on the concentration it have 68% water as the ingredients in the solution. It is commonly prepared by dissolving the zinc with the nitric acid to form the zinc nitrate. In the thermal decomposition it is heated highly and leads to form the zinc oxide, nitrogen dioxide and oxygen.
Zn + 2HNO3 → Zn(NO3)2 + H2
Physical Properties
| Melting point | 110C |
| Boiling point | 125C |
| Molecular weight | 189.36g/mol |
| Density | 2.065g/cm3 |
| Solubility in water | Soluble |
| Refractive index | 1.54 |
| Crystal structure | hexagonal |
| Magnetic susceptibility | -63×10-6cm3/mol |
| Appearance | Colourless, deliquescent crystals |
| Solubility | Very soluble in alcohol |
Chemical Properties
The melting and boiling point of zinc nitrate is equally balanced. It is soluble in the water. it is hexagonal in the structure. It is very soluble in the alcohol. zinc nitrate has a colourless and deliquescent crystals. It is highly harmful substances and a non inflammable compounds. The main hazards is oxidant and may explode on the heating.
Uses
Zinc nitrate is used for dyeing as an mordant. It is a catalyst for resin production. It is also a strong oxidizing agent. It is mainly used for manufacturing of medicines and also a latex coagulant.