Zinc Hydroxide
Zinc hydroxide is an inorganic compound that has the white powder in the appearance. It contains three rare minerals such as wulfingite, ashoverite and sweetite. Zinc hydroxide have the properties of reacting two compounds such as bases and acids. When it is exposed to the strong acid the insoluble hydroxide is dissolved. The systematic IUPAC name is known as zinc hydroxide. The chemical or molecular formula of zinc hydroxide is Zn(OH)2.
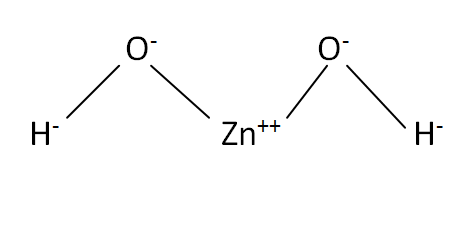
Structural Formula
This is the structural formula of the zinc hydroxide:
Chemical Formula
The chemical formula of the zinc hydroxide is Zn(OH)2.
Preparation Method
The zinc hydroxide is prepared by adding the zinc salt with the sodium hydroxide solution to form. Because the zinc is cations and hydroxide is anions and it formed the equal concentrations. When the precipitation of zinc hydroxide is dissolved the sodium hydroxide is added at a large quantity for leaving the colourless zincate ion solution.
Zn + 2OHB → Zn(OH)2
Physical Properties
| Melting point | 125C |
| Boiling point | decomposes |
| Molecular weight | 99.424g/mol |
| Density | 3.053g/cm3 |
| Solubility in water | Slightly soluble |
| Solubility in alcohol | Insoluble |
| Magnetic susceptibility | -67.1×10-6cm3/mol |
| Appearance | White powder |
Chemical Properties
When the zinc hydroxide is reacted with the aluminium that forms a white precipitate solution that could be indicated the presence of aluminium. It has insoluble in alcohol. It has a low density and medium molecular weight. The magnetic susceptibility is slightly greater.
Uses
Zinc hydroxide is used in medicine as an absorbing agent. It is used in commercial production of pigments and pesticides. They are utilized careful in dressings works at retentive. Huge bandages are used in post medical procedure. Zinc hydroxide is also mainly used agricultural field for pesticides. It reacts with both bases and acids. It is reacts with strong acid and get dissolved. It is medically used in absorbing agent.