Zinc Acetate Formula
Zinc acetate is a salt which is white in colour and are colourless solids. It is commonly the dihydrate of the anhydrous forms by the action of dietary supplements. It was prepared by the action of acetic acid or zinc metal. This is used to adding the reagent of food additive. The systematic IUPAC name is known as zinc acetate. The chemical or molecular formula of acetamide is Zn(CH3COO)2(H2O)2.
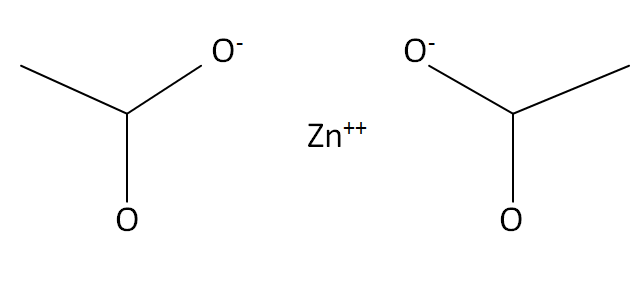
Structural Formula
This is the structural formula of the zinc acetate:
Chemical Formula
The chemical formula of the zinc acetate is Zn(CH3COO)2(H2O)2.
Preparation Method
The reaction of the zinc oxide with the acetic acid to form the zinc acetate. It basically white crystalline in nature. It looks like a cluster bond form that could be the closely resembles the corresponding beryllium compound. Moreover it is polymer structures to give the range of acetate ligands that could be interconnected through the tetrahedral atoms.
Physical Properties
| Melting point | 237C |
| Boiling point | decomposes |
| Molecular weight | 183.468g/mol |
| Density | 1.735g/cm3 |
| Solubility in water | 43g/100mL |
| Crystal structure | tetrahedral |
Chemical Properties
The zinc acetate is a mild toxic and it is not given to the any humans or animals. It is only used for the reactions that could be formed. It is decomposed at the boiling point of 237C. It has highly insoluble in water. However it is the non explosive material and be a slightly non inflammable substances.
Uses
It is widely used in the treatment of acne. It mostly used in itches and rashes. This compound is also used industrially to preserve wood. Zinc acetate is commercially used to produce salts. It is analytical reagent. It mostly used in the important of emetic, astringent, and styptic. It has two form anhydrous and dihydrate form. It is zinc acetate as well as molecular entity. It is used in dimordantand also used in waterproofing agent. Zinc acetate is used in ondirect food additive.