Sodium Sulfide
Sodium sulfide is a chemical compound which has a strong alkaline solution. It is exposed in the moisture air that means it emits the strong rotten eggs smell. By the carthothermic reaction the coal is used to produce the sodium sulfide. They are colourless in the both forms such as anhydrous and hydrated salts. Due to the presence of the polysulfides it have a yellow appearance. The systematic IUPAC name is known as sodium sulfide . The chemical or molecular formula of sodium sulfide is Na2S. It is also known as disodium sulfide.
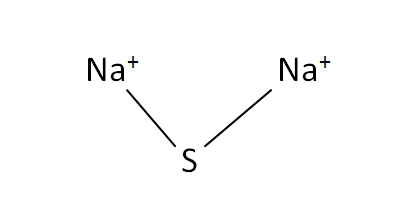
Structural Formula
This is the structural formula of the sodium sulfide:
Chemical Formula
The chemical formula of the sodium sulfide is Na2S.
Preparation Method
There are two methods for preparing the sodium sulfide. They are in the carbothermic reactions the sodium sulfate is reacted with the coal at the moderate temperature and it gives the result of sodium sulfide. Carbon dioxide is emitted as the byproduct. In the another method the sulfur can be reduced to the sodium with the anhydrous ammonia it could be the formed the sodium sulfide. The naphthalene is used as the catalyst substances.
Na2SO4 + 2C → Na2S + 2CO2
2Na + S → Na2S
Physical Properties
| Melting point | 1176C |
| Boiling point | decomposes |
| Molecular weight | 78.0452g/mol |
| Density | 1.86g/cm3 |
| Solubility in water | Soluble |
| Crystal structure | Antifluorite (cubic) |
| Magnetic susceptibility | -39.0×10-6cm3/mol |
| Appearance | Colourless hydroscopic solid |
| Solubility | Insoluble in ether
Slightly soluble in alcohol |
Chemical Properties
Sodium sulfide has a highly harmful substances that contains the autoignition temperature over 480C. it is more hazardous to the environment. The melting point is very high and decomposed at the boiling point. It is insoluble in ether and slightly soluble in alcohol. It is antifluorite in structure. It has low density and moderate molar mass. It looks like a colourless hydroscopic in the appearance.
Uses
Sodium sulfide is mainly used for photography and bleaching powder for textile industry. In paper industry they manufacturing a pulp in craft process. It is widely used for water treatment as an oxygen scavenger agent. In leather processing it is used an unhairing agent.