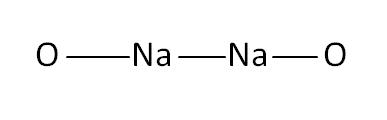Sodium Peroxide
Sodium peroxide is an inorganic compound that is mixed with the combustible substances for heating by the process of friction. It is ignited when the excess of oxygen is occurred. Sodium peroxide is a strong base acid. This will goes to the transition phase with the unknown symmetry of the hexagonal form. It is soluble in the hydrates and metal peroxide materials. The systematic IUPAC name is known as sodium peroxide . The chemical or molecular formula of sodium peroxide is Na2O2. It is also known as disodium dioxide or solozone.
Structural Formula
This is the structural formula of the sodium peroxide:
Chemical Formula
The chemical formula of the sodium peroxide is Na2O2.
Preparation Method
When the sodium hydroxide is reacted with the hydrogen peroxide to form the sodium peroxide. At the moderate temperature it absorbs the oxygen by the reaction of metallic sodium. In mild heating the iodine can be sublimed. These reactions is catalyzed by the platinum or palladium crystals. In another method it is produced by passing the ozone gas on the palladium or platinum tube which is present is called sodium iodide.
2 NaOH + H2O2 → Na2O2 + 2H2O
Physical Properties
| Melting point | 460C |
| Boiling point | 657C |
| Molecular weight | 77.98g/mol |
| Density | 2.805g/cm3 |
| Solubility in water | Reacts |
| Crystal structure | hexagonal |
| Magnetic susceptibility | -28.10×10-6cm3/mol |
| Appearance | Yellow to white powder |
| Solubility | Soluble in acid
Insoluble in base |
Chemical Properties
Sodium peroxide is looks a yellowish solid in the appearance. It reacts with the soluble in water. it is hexagonal in the structure. It is insoluble in base substances. sodium peroxide is a non inflammable substances and hazardous to the environment.
Uses
Sodium peroxide is a bleaching agent. It is widely used in chemical laboratories and also the production of other chemicals. Sodium peroxide can make the wood pulp for the production of paper and textiles.