Sodium Nitrite
Sodium nitrite is an inorganic compound that has contained the strong reactive agent which is reacted with the bacterias in the food as a food preservative. sodium nitrite is very soluble and hygroscopic in nature. It should be the more effective for the manufacturing the chemicals at the industrial perspective. Moreover these salt has the ability to destroy the poisoning substances in the food raw materials. The systematic IUPAC name is known as sodium nitrite . The chemical or molecular formula of sodium nitrite is NaNO2.
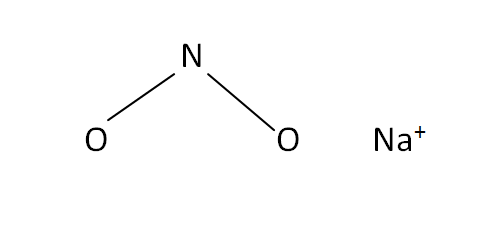
Structural Formula
This is the structural formula of the sodium nitrite:
Chemical Formula
The chemical formula of the sodium nitrite is NaNO2.
Preparation Method
In the aqueous solution, the nitrogen oxides is used to oxidant the nitrogen atoms to produce the sodium nitrite. This is the one of the method for manufacturing the nitrite. In the another method, the nitrate salts has been exposed by the process of heating, ionizing the radiation, lighting and electrolytic reductions. These sodium nitrite is used to destroy the sodium azide in the laboratory.
2 NaNO 2→ Na2O+ NO + NO2
2NaN3 + 2NaNO2 + 4H+ → 3 N2 + 2NO + 4 Na+ + 2H2O
Physical Properties
| Melting point | 271C |
| Boiling point | Decomposes at 320C |
| Molecular weight | 68.9985g/mol |
| Density | 2.17g/cm3 |
| Solubility in water | 71.4 g/100mL (0C) |
| Refractive index | 1.65 |
| Crystal structure | Orthorhombic |
| Magnetic susceptibility | -14.5×10-6cm3/mol |
| Appearance | White or slightly yellowish solid |
Chemical Properties
Sodium nitrite is the more harmful and inflammable substances. it is more hazardous to the environment. It is white or slightly yellowish solid in the appearance. It looks like an orthorhombic in structure. The melting point is very low and the boiling point decomposed at above 320C.
Uses
Sodium nitrite is used as a humane toxin for wild boar control. It is necessity as a food additive. It is responsible for desirable red colour in meat. It has an effective result for corrosion inhibitor.