Sodium Nitride
Sodium nitride is an inorganic compound and have bonded with the carbon-hydrogen atoms. It is an alkali metal and have the group 1 in the periodic table. It has the combination of sodium and nitrogen at the point of sapphire with the low temperatures. The sodium has the positive ions and the nitride has the negative ions. It is ability to decomposed at the level of temperature is decreased or increased in the threshold limits. The systematic IUPAC name is known as a sodium nitride . The chemical or molecular formula of sodium nitride is Na3N.
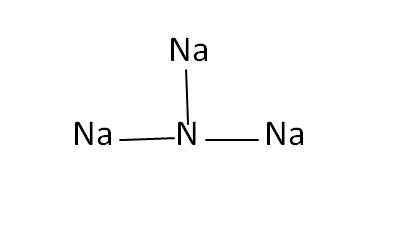
Structural Formula
This is the structural formula of the sodium nitride:
Chemical Formula
The chemical formula of the sodium nitride is Na3N.
Preparation Method
It can be prepared by reacting with the nitrogen and sodium at the vaccum chamber on the cooled substrate. It gives the result of sodium nitride. It can be synthesiszed by the two ways. One is thermal decomposition and other one is direct reaction. At the desired ratios the Na and N kept separately in the different chambers to produce the sodium nitride. In another way the metal surface is activated on the plasma nitrogen to react the element of the sodium.
2 Na3N → 6 Na + N2
Physical Properties
| Melting point | 87C |
| Boiling point | 452C |
| Molecular weight | 82.98g/mol |
| Density | 2.17g/cm3 |
| Solubility in water | Soluble |
| Refractive index | 1.559 |
| Crystal structure | Cubic |
| Magnetic susceptibility | -14.7.1×10-6cm3/mol |
| Appearance | Reddish brown or dark blue solid |
Chemical Properties
Sodium nitride has the equivalent bandgap to the semiconductors and it contains the 90% ionic when it is at room temperature. It has high boiling and low melting point. Due to the electrostatic reaction it could be charged oppositely. It is soluble in the water. it looks reddish brown or dark blue solid in the appearance.
Uses
Sodium nitride is used as a food preservative and also as a substitute for salt. It is required for antidote to cyanide poisoning. It is a microbial agent for fish and meat.