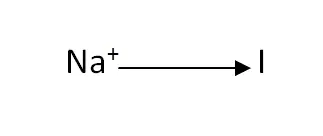Sodium Iodide
sodium iodide is an iconic chemical compound which is necessity for making the salts by producing the chemical reaction between the sodium and iodide metal. The sodium is the positively charged and the anion is the negatively charged particles. It supplies the nutritional supplement and used in the organic chemistry. It is mixture in the ratio of 1:1 in the cubic lattice. The systematic IUPAC name is known as sodium iodide. The chemical or molecular formula of sodium iodide is NaI.
Structural Formula
This is the structural formula of the sodium iodide:
Chemical Formula
The chemical or molecular formula of sodium iodide is NaI.
Preparation Method
When the sodium hydroxide is reacted with the hydroxide acid or iodine and it gives the result of the formation of sodium iodide. It accelerates the oxidation process and iodide can produce the photooxidation with the low temperature and humidity conditions. It is a well known nutritional salt that has contains the more minerals with the high carbonic content.
NaOH + HI → NaI + H2O
Physical Properties
| Melting point | 661C |
| Boiling point | 1304C |
| Molecular weight | 149.89g/mol |
| Density | 3.67g/cm3 |
| Solubility in water | Soluble |
| Refractive index | 1.93 |
| Crystal structure | Halite |
| Magnetic susceptibility | -57×10-6cm3/mol |
| Appearance | White solid deliquescent |
| Odor | odourless |
Chemical Properties
Sodium iodide is a more hazards to all the living creatures. The main hazards is irritant the skin, eyes to the humans and also harmful to the unborn child. It has high boiling point and odourless things. The refractive index is less than the 2 point. It is insoluble in acetone and ethanol. It looks like a halite in structure.
Uses
Sodium iodide is widely used for medicine in the treatment of tyroid. It is also made the treatment of actinobacillosis and actinomycosis in cattle. A huge amount of sodium iodide is added to sodium chloride to prepare the salts.