Sodium Hydrogen Phosphate
Sodium hydrogen phosphate is an organic compound that has the composition of the sodium and hydrogen. It is the form of anhydrous such as form of hydrates. It is basically oxidizing agent. It is being in the hydroscopic and all are water soluble powders. It is partially neutralized and obtained the monosodium phosphate solutions. The systematic IUPAC name is known as sodium hydrogen phosphate. The chemical or molecular formula of sodium hydrogen phosphate is Na2HPO4. It is also known as disodium phosphate.
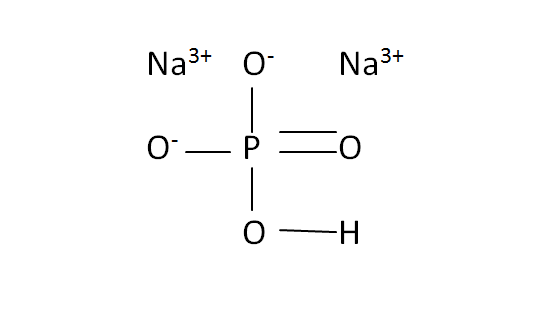
Structural Formula
This is the structural formula of the sodium hydrogen phosphate:
Chemical Formula
The chemical formula of the sodium hydrogen phosphate is Na2HPO4. It has consists of two sodium ions, four oxygen ions, one phosphorous and one oxygen.
How it is Produced
It has prepared by the two methods. First method, when the phosphoric acid is reacted with the sodium hydroxide it gives the formation of the sodium hydrogen phosphate as the product and water is the byproduct.
H3PO4 + 2 NaOH → Na2HPO4 + 2H2O
In the second method, the dicalcium phosphate with sodium bisulfate can be reacted and it is formed the monosodium phosphate. Then it is neutralized with the sodium hydroxide and gives the sodium hydrogen phosphate.
NaH2PO4 + NaOH → Na2HPO4 + H2O
Physical Properties
| Melting point | 250C |
| Boiling point | Decomposes |
| Molecular weight | 141.96g/mol |
| Density | 1.7g/cm3 |
| Solubility in water | 7.7g/100mL |
| Refractive index | 1.35664 |
| Crystal structure | Halite (cubic) |
| Magnetic susceptibility | -56.6×10-6cm3/mol |
| Appearance | White crystalline solid |
| Solubility | Insoluble in alcohol. |
Chemical Properties
Sodium dihydrogen phosphate is a non inflammable substances. It is irritant to the eyes and skins. It is decomposed at the boiling point. The refractive index is less than the vapour pressure. It appears as a white crystalline solid. it is insoluble in the alcohol.
Uses
Sodium hydrogen phosphate is mainly used for food and water treatment. It is a thickening and leavening agent. It is used as an emulsifiers.