Sodium Hydrogen Carbonate
Sodium hydrogen carbonate is a chemical compound that has consists of a sodium cation and a bicarbonate anion. The mineral has a slightly alkaline taste but offers appears as a fine powder. It is weakly basic oxidizing agent. It is an alkali metal salt and has a high reactivity with water. The systematic IUPAC name is known as sodium hydrogen carbonate. The chemical or molecular formula of sodium hydrogen carbonate is NaHCO3. It is also known as baking soda or bicarbonate of soda.
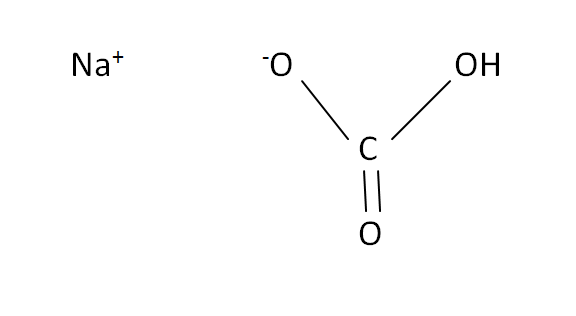
Structural Formula
This is the structural formula of the sodium hydrogen carbonate:
Molecular Formula
The chemical formula of the sodium hydrogen carbonate is NaHCO3. It has consists of one sodium and two oxygen atoms.
How it Can Be Prepared
It can be prepared by the reaction of sodium chloride is treated along with the water and carbon dioxide and it gives the product of sodium bicarbonate and byproduct is the hydrochloric acid. It is an alkaline and could be used in the baking powder to avoid a metallic taste. It slowly reacts at the room temperatures. It can be decomposed at above the threshold limit. The following chemical reaction is given as follows.
Na2CO3 + H2O + CO2 → 2 NaHCO3 + HCl
Physical Properties
| Melting point | 50C |
| Boiling point | Decomposes |
| Molecular weight | 84.006g/mol |
| Density | 2.20g/cm3 |
| Solubility in water | 69g/100mL |
| Refractive index | 1.377 |
| Crystal structure | Monoclinic |
| Magnetic susceptibility | -15.6×10-6cm3/mol |
| Appearance | White crystals |
| Solubility | Insoluble in alcohol |
| Odor | Odourless |
| Acidity | 6.34 |
Chemical Properties
Sodium bicarbonate is an incombustible organic compounds. It avoids the corrosive inhibitor to the metals. It leads to severe eye irritation when it is contact with the humans. It appears as white crystals. It is insoluble in alcohol. The melting point is very low and it decomposed at the boiling point.
Applications
Sodium hydrogen carbonate is used as a disinfectant. It is used in medicine prevention of chemotherapy and also used for washing the kitchen products. It is mainly used for cooking. Sodium hydrogen carbonate is used to clean the mouth and teeth.