Sodium Fluoride
Sodium fluoride is the chemical organic salt that is used to fluoridation of drinking water, metallurgy, toothpaste etc. sodium fluoride has considered as one atom of sodium and one atom of fluorine. It can be precipated in the alcohols. It acts as the corrosive resistant for the aluminium metals. It could be easily dissolved in all other chemicals.The systematic IUPAC name is known as magnesium oxide . The chemical or molecular formula of magnesium oxide is MgO. It is also known as florocid or pediaflor.
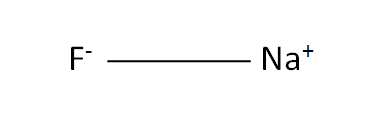
Structural Formula
This is the structural formula of the sodium fluoride:
Chemical Formula
The chemical formula of the sodium fluoride is NaF.
Preparation Method
This sodium fluoride is prepared by the neutralizing process. When the hydrofluoric acid is reacted with the sodium hydroxide it forms the sodium fluoride is the product and water as the byproduct. Because the alcoholic substances are easily dissolved. It occupies at the octahedral coordination sites. The sodium has positively charged and fluoride has negatively charged. The following chemical reaction is given as follows.
HF + NaOH → NaF +H2O
Physical Properties
| Melting point | 993C |
| Boiling point | 1704C |
| Molecular weight | 41.988173g/mol |
| Density | 2.558g/cm3 |
| Solubility in water | 36.4g/100mL(0C) |
| Refractive index | 1.3252 |
| Crystal structure | Cubic |
| Magnetic susceptibility | -16.4×10-6cm3/mol |
| Appearance | White to greenish solid |
| Solubility | Slightly soluble in HF and ammonia. |
| Odor | Odourless |
| Molecular shape | octahedral |
Chemical Properties
Sodium fluoride is a non inflammable substances. It is salinity in taste. It converts the white to greenish solid when the chemical reaction is occurs. It is cubic in the structure . The molecular shape of sodium fluoride is octahedral. The boiling point is high and the melting point is low. It is hazardous to the environment.
Uses
Sodium fluoride is used in the nuclear molten salt reactors. With the help of laundry soul it acts as a cleansing agent. It was used as stomach poison for the plant feeding insects. It prevents from spreading poisonous substances in the oxidative metabolism.