Sodium Dihydrogen Phosphate
Sodium dihydrogen phosphate is an inorganic compound which is get derived from the edible sources with the phosphorous pentoxide. It has consists of three hydrogens and four oxygen atoms. If the electronic configuration of the atoms is combined with the calcium, phosphorous and magnesium. The systematic IUPAC name is known as sodium dihydrogen phosphate . The chemical or molecular formula of sodium dihydrogen phosphate is NaH2PO4. It is also known as monosodium phosphate or monobasic sodium phosphate.
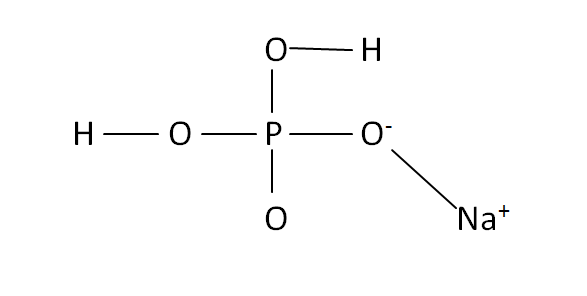
Structural Formula
This is the structural formula of the sodium dihydrogen phosphate:
Chemical Formula
The chemical formula of the sodium dihydrogen phosphate is NaH2PO4.
How it Can be Prepared
It can be prepared by the process of calcination. When the sodium hydrogen phosphate is react along with the water, it produces the sodium dihydrogen phosphate is product and sodium hydroxide is the byproduct. Otherwise it also reacts with the hydrochloric acid and formed the phosphoric acid and sodium chloride. The chemical reaction is given as follows.
Na2HPO4 + H2O → NaH2PO4 +NaOH
Physical Properties
| Melting point | Nil |
| Boiling point | Nil |
| Molecular weight | 119.98g/mol |
| Density | 2.36g/cm3 |
| Solubility in water | 59.90g/100mL(0C) |
| Appearance | White powder or crystals |
Chemical Properties
Sodium dihydrogen phosphate is a white powder or crystals. It is a strongly base chemical compounds. But it reacts with the both acid and basic forms. It has low density and high molecular weight. There is no melting point and boiling point. It has bitter in taste.
Applications
Sodium dihydrogen phosphate is used to detect the magnesium ions in the salt. It contains ph buffer in baking powder. It adds as the sequestrant in the food. In the boiled water treatment it is mainly used for cleaning process. It has a thickening and emulsifier so it is added for toothpastes, animal feeds and evaporated milk.