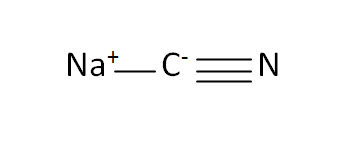Sodium Cyanide
Sodium cyanide is a highly toxic chemical compounds which has the high affinity points considered as one of the main substances. It is a water soluble salt and highly reacted towards the metals. It has a strong base agent. It exploits the high reactivity when it is used in the goldmine industry. It has contain a single bonded and three bonded structure. The atoms are separated with the electrons by the electronic configuration. The systematic IUPAC name is known as sodium cyanide . The chemical or molecular formula of sodium cyanide is NaCN.
Structural Formula
This is the structural formula of the sodium cyanide:
Chemical Formula
The chemical formula of the sodium cyanide is NaCN.
How it is Prepared
When the hydrogen cyanide is reacted with the sodium hydroxide and it gives the formation of sodium cyanide. It can be synthesized in the castner process. At the moderate temperature it is reacted with the carbon to produce the sodium cyanide and evolved the hydrogen gas. It smells like a faint almonds. Both the cations and anions can contain the six coordinate each. The following chemical reaction is given as follows.
HCN+ NaOH → NaCN +H2O
NaNH2 + C → NaCN + H2
Physical Properties
| Melting point | 563.7C |
| Boiling point | 1496C |
| Molecular weight | 49.008g/mol |
| Density | 1.5955g/cm3 |
| Solubility in water | 63.7g/100mL(25C) |
| Refractive index | 1.452 |
| Appearance | White solid |
| Solubility | Soluble in ammonia, methanol and ethanol. Insoluble in dimethyl sulfoxide. |
| Odor | Faint almond like |
Chemical Properties
Sodium cyanide is a non inflammable and highly toxic chemical compounds. It is insoluble in dimethyl sulfoxide. The refractive index is less than the threshold limit. It appears as a white solid. The boiling point is high and the melting point is low. It has low density and low molecular weight.
Applications
Sodium cyanide is used to extract the gold as well as precious other metals from the mines. In the organic synthesis it helps to preparing the nitriles and it is used in pharmaceutical industry. These chemicals are kills the insects and rodents. It is used to clean the rust iron metals.