Sodium Citrate
Sodium citrate is a chemical compound that is used to adding flavor to food productive materials in the industry. E331 is the most additive flavouring materials. It dissociates to the sodium cations and citrate anions. It also acts as a anticoagulant agent materials.it also acts as a anitcoagulant agents. The systematic IUPAC name is known as sodium citrate. The chemical or molecular formula of sodium citrate is Na3C6H5O7.
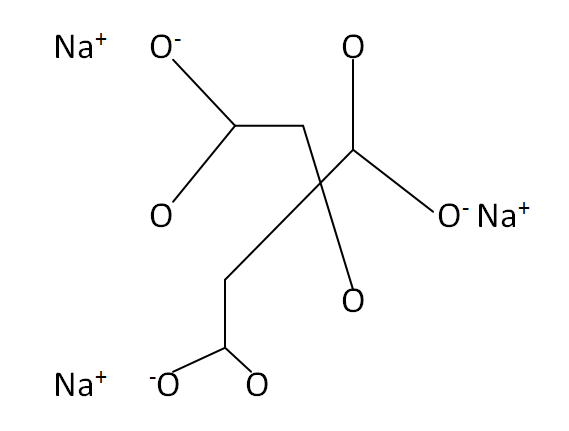
Structural Formula
This is the structural formula of the sodium citrate:
Chemical Formula
The chemical formula of the sodium citrate is Na3C6H5O7.
Preparation method
Sodium citrate is prepared with the help of the reacting with the sodium bicarbonate as the adding agent.by the absorption process it looks like a little alkanizing activity to the form of materials. It has more common salt quality reactions in the redox oxidasation methods.
Physical Properties
| Melting point | 300C |
| Boiling point | 309.6C |
| Molecular weight | 258.06g/mol |
| Density | 1.7g/cm3 |
| Solubility in water | soluble |
Chemical Properties
This sodium citrate has high boiling point and more hazardous substances. In organic synthesis it can be used to produce the other citrate chemical compounds. It has a cumulative toxic with a relatively half long life. It looks like a more severe toxic substances compared to the other chemical compound
Uses
Sodium citrate is used in emulsifier of oils. It is also used in medical field for pharmautical aid. sodium citrate is enables the melting of cheeses. It is widely used infood additive. It prevent the curdling of milk. It used to avoid clotting of blood. It is mainly used as an elactroplating. Usually used in foods and drinks as an acidity regulator. sodium citrate is used in mainly oil industry.