Sodium Chromate
Sodium chromate is an inorganic compound that is used to corrosion inhibitor in the petroleum industry. It is naturally occur in the yellow hygroscopic solid. It is extracted from its ore and comprised to the form of tetra hexa. It is more toxic substances and it delivers the carcinogenal compounds substances. It has converting to the dissolved compounds. The systematic IUPAC name is known as sodium chromate. The chemical or molecular formula of sodium chromate is Na2CrO4.
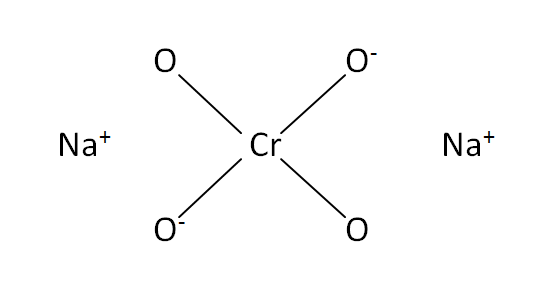
Structural Formula
This is the structural formula of the sodium chromate:
Chemical Formula
The chemical formula of the sodium chromate is Na2CrO4.
Preparation Method
When the chromium ores is roasted with the sodium carbonate in the presence of air it gives the result of sodium chromate. The iron oxides is leaved behind when the process of sodium chromate is converted to the water extractable form. It occurs around 1100 degree Celsius temperature. Simultaneously this chromite ore is extracted and mixture with the sodium hydroxide and sodium nitrate.
2Cr2O3 + 4 Na2CO3 + 3O2 → 4 Na2CrO4 + 4 CO2
Physical Properties
| Melting point | 782C |
| Boiling point | 400C |
| Molecular weight | 161.97g/mol |
| Density | 2.698g/cm3 |
| Solubility in water | 126.7g/100mL(100C) |
| Crystal structure | orthorhombic |
| Magnetic susceptibility | +55.0×10-6cm3/mol |
| Appearance | Yellow crystals |
Chemical Properties
In the acid base behavior when it is treated with the acids it converts to the sodium dichromate. If the further acidification is occurred it converts to the chromium trioxide. It is used as a oxidant that means it converting the primary alcohols to carboxylic acid and secondary alcohols to ketones.
Uses
Sodium chromate is in yellow crystalline odourless. This is mainly used in inks,paints and wood preservative. sodium chromate is widely used in making of glass. It plays a major in manufacturing of orgnic compouds like esters. sodium chromate is mostly found in living environment. sodium chromate is used in structure of alloys and molten metals purification. It leaves the pressure of fingers.