Sodium Chlorate
Sodium chlorate is an inorganic compound that has the consists of the sodium and chlorate. Yearly it produces the millions of tones for using the many applications in the industry oriented. At the temperature of above 300C it releases the oxygen. It is hygroscopic in nature. It converts from the pale yellow to white crystalline solid.The systematic IUPAC name is known as sodium chlorate . The chemical or molecular formula of sodium chlorate is NaClO3. It is also known as sodium chlorate (V).
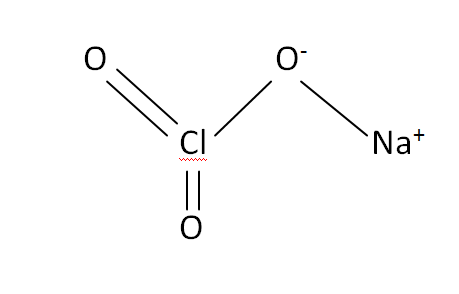
Structural Formula
This is the structural formula of the sodium chlorate:
Molecular Formula
The chemical or molecular formula of the sodium chlorate is NaClO3.
How the Sodium Chlorate is Prepared
The sodium chlorate can be prepared by the electrolysis concentrated reaction. When the sodium chloride is reacted with the water it will produces the sodium chlorate as the product and hydrogen as the byproduct. In this process the sodium acts as the cathode and chloride acts as the anode. It transfers the free electrons along with the reaction between these two chemicals. The chemical reaction is as follows.
NaCl + 3H2O → NaClO3 +3H2
Physical Properties
| Melting point | 248C |
| Boiling point | 300C |
| Molecular weight | 106.44g/mol |
| Density | 2.5g/cm3 |
| Solubility in water | Soluble |
| Refractive index | 1.515 |
| Crystal structure | Cubic |
| Magnetic susceptibility | -34.7×10-6cm3/mol |
| Appearance | Colourless or white solid, hygroscopic |
| Solubility | Soluble in glycerol, hydrazine, methanol. |
| Odor | Odourless |
| Vapour pressure | <0.35mPa |
Chemical Properties
Sodium chlorate is a non inflammable substances. It is very danger to the environment. It is slightly soluble in water. The structure it contains is cubic . It looks like a colourless or white solid in the appearance. The vapour pressure of the sodium chlorate is less than 1. It is bitter in taste.
Applications
Sodium chlorate is commercially used for manufacturing the chlorine dioxide. It kills the root absorption in the agriculture. It is making the paper and pulp industries. It acts as the chlorination agents in the organic synthesis.