Silver Phosphate
Silver phosphate is a light sensitive and water insoluble chemical compound that has been combination of silver and phosphate ions. The silver has positively charged ions and phosphate has negative charged ions. It can be dissolved in the aqueous ammonia. Gradually it evaporates from that ammoniacal solutions. The systematic IUPAC name is known as silver phosphate. The chemical or molecular formula of silver phosphate is Ag3PO4. It is also known as argentous phosphate or phosphoric acid.
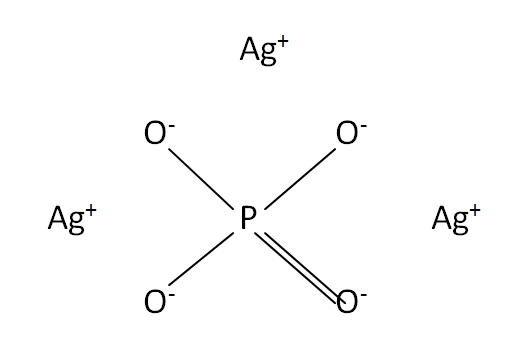
Structural formula
This is the structural formula of the silver phosphate:
Molecular formula
The chemical or molecular formula of the silver phosphate is Ag3PO4. It has consist of silver and phosphate ions.
Preparation method
When the silver nitrate is reacted with the orthophosphate which is called as the salt that will be formed the silver phosphate is the product. It is thermally decomposed at the room temperatures. At the end of the reaction it can be precipitated along with the endothermic reaction. The chemical reaction can be given as follows.
AgNO3 + PO43- → Ag3PO4
Physical properties
| Melting point | 849C |
| Boiling point | Decomposed |
| Molecular weight | 418.574g/mol |
| Density | 6.370g/cm3 |
| Solubility in water | 0.00065 g /100mL |
| Refractive index | 1.53 |
| Crystal structure | Cubic |
| Magnetic susceptibility | -120.0×10-6cm3/mol |
| Appearance | Translucent yellow becomes opaque or dissolves when impure. |
| Solubility | Soluble in acid, ammonia. Insoluble in alcohol. |
| Odor | odourless |
Chemical Properties
The boiling point is decomposed and the melting point is low. Silver phosphate is an odourless chemical compound. It is cubic in the structure. It is soluble in acid and ammonia. The magnetic susceptibility is high. It looks like a translucent yellow in the appearance. It has low density and moderate molar mass.
Uses
Silver phosphate is a polishing agent used for toothpaste. It is manufacturing the pharmaceuticals and personal products. It is widely for fire extinguishers. It is usually used in industrial and institutional cleaners.