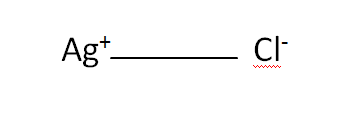Silver Chloride
Silver chloride is a chemical compound and it have low solubility in the water. By heating or illumination it can be convert to the silver from silver chloride which is known as grey to black or purplish in the colour. These silver chloride is naturally occurred from the chlorargyrite. It should be the reminiscent in the behavior. The systematic IUPAC name is known as silver chloride. The chemical or molecular formula of silver chloride is AgCl. It is also known as horn silver or argentous chloride.
Structural Formula
This is the structural formula of the silver chloride:
Chemical Formula
The chemical or molecular formula of the silver chloride is AgCl. It has consists of silver and chloride ions.
How Can be Produced
It can be easily synthesized by the metathesis process. In this process the silver nitrate is reacted with the sodium chloride usually it means a chlorine salt and gives the result of silver chloride as the product and sodium nitrate as the byproduct. The solubility of the silver chloride is very low. Immediately it will be precipitated at any given solution. The chemical reaction is given as follows.
AgNO3 + NaCl → AgCl + NaNO3
Physical Properties
| Melting point | 455C |
| Boiling point | 1547C |
| Molecular weight | 143.32g/mol |
| Density | 5.56g/cm3 |
| Solubility in water | Soluble |
| Refractive index | 2.071 |
| Crystal structure | Halite |
| Magnetic susceptibility | -49.0×10-6cm3/mol |
| Appearance | White solid |
| Solubility | Soluble in NH3, conc HCl, H2SO4. Insoluble in alcohol,dilute acids. |
Chemical Properties
Silver chloride is a non inflammable substances. It is quickly exposure by light to disintegrate the chlorine and metallic silver. It is a non hygroscopic solid. The boiling point is high and the melting point is low. The refractive index of the silver chloride is high. It is halite in structure. It looks like a white hygroscopic solid in the appearance.
Uses
Silver chloride is used for electroplating and polishing mirrors and making alloys. It is mainly used in medicines and also used for photographic films. It has been activated battery usage for magnesium.