Potassium Sulfate
Potassium sulfate is an inorganic compound with the combination of the potassium and sulfate. It has a water soluble solid. potassium sulfate is a natural resources that has found abundant naturally. It is less soluble in water. These minerals can be separated from the other minerals like kainite which is the primary sources of the potassium chemicals. The systematic IUPAC name is known as potassium sulfate. The chemical or molecular name of potassium sulfate is K2SO4. It is also known as sulfate of potash.
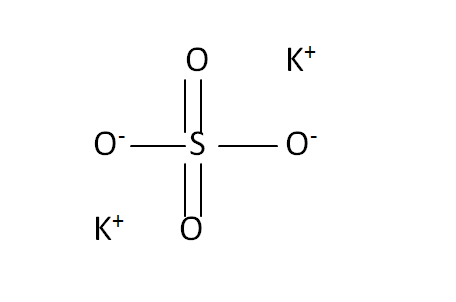
Structural Formula
This is the structural formula of the potassium sulfate:
Molecular Formula
The chemical formula of the potassium sulphate in K2SO4. It has consists of two potassium ions and four oxygen atoms and one sulfur compounds.
How the Potassium Sulfate is Prepared
The potassium sulfate can be prepared by the Glauber’s process. In the Glauber’s process , potassium nitrate is reacted with the sulfuric acid that is formed potassium sulfate as the product and nitric acid as the byproduct. Then it has been dissolved in hot water, filtered and evaporated to a cuticle. It can also prepared by the endothermic process, when the potassium chloride is reacted with the potassium hydrogen sulfate and it gives the result of potassium sulfate.
2 KNO3 + H2SO4 → K2SO4 + 2 HNO3
Physical Properties
| Melting point | 1689C |
| Boiling point | 1069C |
| Molecular weight | 174.269g/mol |
| Density | 2.66g/cm3 |
| Solubility in water | 111g/100mL |
| Refractive index | 1.495 |
| Crystal structure | Orthorhombic |
| Magnetic susceptibility | -67.0×10-6cm3/mol |
| Appearance | White solid |
| Solubility | Slightly soluble in glycerol. Insoluble in acetone and alcohol. |
Chemical Properties
Potassium sulfate is a non inflammable substances. It is more hazardous to the environment. It has more molecular weight and less density. It looks like a orthorhombic in structure. it appears as a white solid crystals. The melting point is high and the boiling point is low. It is insoluble in acetone and alcohol.
Applications
Potassium sulphate is used for soda blasting. It is mainly used for making ceramics and glasses. Potassium sulphate is used as a salt substitute. It is widely used in the production of gypsum board and also a gypsum cement. It is used to supplement the animal feeds.