Potassium Oxide
Potassium oxide is an ionic chemical compound which is made up of potassium and oxygen. Its valency has a high tendency to cover the group of alkali metals. Potassium has described as an oxidation state whether the oxygen is described as reduction state. The alkali metals are easily dissolved in the water. The systematic IUPAC name is known as potassium oxide . The chemical or molecular formula of potassium oxide is K2O. It is also known as the potash.
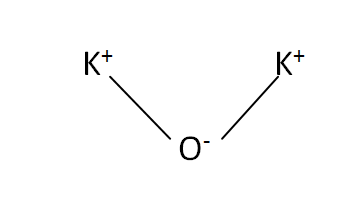
Structural Formula
This is the structural formula of the potassium oxide:
Chemical Formula
The chemical formula of the Potassium oxide is K2O.
Preparation Method
The potassium oxide is prepared by the reaction of potassium peroxide with potassium and gives the formation of results given above. In another method it is synthesized by the potassium nitrate with the metallic potassium to form the potassium oxide. Because it reacts with the water violently and easily absorbed. It has contributed the 4 oxide ions to the coordinated of potassium metals.
K2O2 + 2K → 2K2O
Physical Properties
| Melting point | 740C |
| Boiling point | 300C |
| Molecular weight | 94.2g/mol |
| Density | 2.35g/cm3 |
| Solubility in water | Reacts forming KOH |
| Odor | odourless |
| Crystal structure | Antifluorite cubic |
| Solubility | Soluble in diethyl ether |
| Appearance | Pale yellow solid |
Chemical Properties
The potassium oxide has a low melting and boiling point. It reacts to form the KOH. It looks like a pale yellow solid in the appearance. It is soluble in diethyl ether. It is odourless and antifluorite in structure. It has a low density and moderate molar mass.
Uses
Potassium oxide is used for farming fertilizer. It is also making for the glass and soaps. It is widely treatment in medical purposes. Potassium oxide is used in the treatment of actinomycosis and actinobacillosis. It cures the fungal infections such as zygomycetes and animal related diseases.