Potassium Nitrate
Potassium nitrate is a chemical compound which is a white solid substances that has soluble in water. It is crystallized with the sodium nitrate and potassium chloride solutions. When the potassium nitrate is heated over it decomposed and obtained the particles such as nitrite and oxygen. It is a non deliquescent in nature. It is generally obtained in the rocks from the various parts of India. The systematic IUPAC name is known as potassium nitrate. The chemical or molecular formula of potassium nitrate is KNO3. It is also known as nitrate of potash.
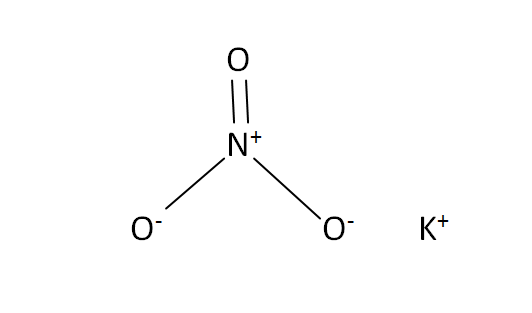
Structural Formula
This is the structural formula of the potassium nitrate:
Chemical Formula
The chemical formula of the potassium nitrate is KNO3.
Preparation Method
When the potassium hydroxide is reacted with the nitric acid that forms the result of potassium nitrate. The hydrogen gas is leaved as the byproduct. The potassium nitrate is a light, soft and silver metal. It has consitsts of odorless and colourless gas such as oxygen and nitrogen. The potassium has cations and the nitrate ions has anions that formed together the potassium nitrate. It is a strong oxidizing agent it released the excess of oxygen when the process of decomposed or heated.
KOH + HNO3 → KNO3 + H2O
Physical Properties
| Melting point | 334C |
| Boiling point | 400C |
| Molecular weight | 101.1032g/mol |
| Density | 2.109g/cm3 |
| Solubility in water | 0.00064mg/L |
| Refractive index | 1.5604 |
| Crystal structure | Orthorhombic, aragonite |
| Magnetic susceptibility | -33.7×10-6cm3/mol |
| Appearance | White solid |
| Basicity | 15.3 |
| Solubility | Slightly soluble in ethanol, soluble in glycerol and ammonia |
Chemical Properties
It is more hazardous and non inflammable. It can be treated as the antacids which is combined in the form of nitric acid and to produce the formation of potassium solution. It helps to improve the techniques and solutions.
Uses
It is used to manufacturing the cigarettes. It is used as a gunpowder in explosives such as bombs, explosives at roctery engine. It is used to the preservation of hides. In the toothpaste it gives the less pain sensitive to make the stronger teeth. In medically it is used to treatment for the asthma.