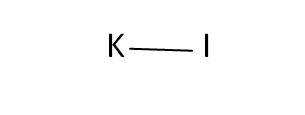Potassium Iodide
Potassium iodide is an ionic compound which has the compostion of potassium and iodine. It looks lika a white crystalline salt and used for the major treatment in the medical fields. The potassium has positive ions and the iodine has the negative ions. Whenever it is achieved the stable it could be the gain or loss in the valency bond. The systematic IUPAC name is known as potassium iodide . The chemical or molecular formula of potassium iodide is KI.
Structural Formula
This is the structural formula of the potassium iodide:
Chemical Formula
The chemical formula of the Potassium iodide is KI.
Preparation Method
It is prepared by the combination of potassium hydroxide and iodide in the formation of condensed stage. Then it is added to the required flavor to create the fully diagonostic minerals. It is simply used in the medical fields such as hyperthyroidism, thyroid gland, phycomycosis and sporotrichosis. The chemical reaction is given as follows.
2 KOH + I2 → 2 KI + H2O
Physical Properties
| Melting point | 681C |
| Boiling point | 1330C |
| Molecular weight | 166.0028g/mol |
| Density | 3.12g/cm3 |
| Solubility in water | Soluble |
| Refractive index | 1.435 |
| Crystal structure | Monoclinic |
| Magnetic susceptibility | -22.1×10-6cm3/mol |
| Appearance | White crystalline salt |
Chemical Properties
Potassium iodide is a white crystalline salt and monoclinic in structure. The boiling point is very high when compared to the melting point. The range of the refractive index is more than the basicity level. It is soluble in water. it has moderate molar mass and low density.
Uses
Potassium iodide is used in the treatment of hyperthyroidism. It helps in hormonal balance. It reduced the loss of iodine in salts. It is widely used for table salt of most common additive. potassium iodide can making an expectorant to break up the mucus.