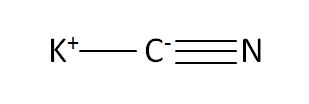Potassium Cyanide
Potassium cyanide is a more toxic substance and a very poisonous inorganic salt to be considered as the more exposure. It can be extracted from the gold and the ores. It is highly soluble in water. The following process is performing such as electroplating, chemical gilding, fumigation and buffing. The elements of the cyanide is used for the lethal for the humans. The systematic IUPAC name is known as potassium cyanide. The chemical or molecular formula of potassium cyanide is KCN.
Structural Formula
This is the structural formula of the potassium cyanide:
Chemical Formula
The chemical formula of the potassium cyanide is KCN.
Preparation Method
When the potassium hydroxide is reacted with the hydrogen cyanide and it gives the formatiom of potassium cyanide. The chemical reaction is follows as
HCN + KOH → KCN+ H2
That means it is made up of solid base and weak acid. In the castner process it can be decomposed the potassium ferrocyanide. Because it has a highly toxic substances.
Physical Properties
| Melting point | 634C |
| Boiling point | 1625C |
| Molecular weight | 65.12g/mol |
| Density | 1.52g/cm3 |
| Solubility in water | 162.9g/100mL(100C) |
| Refractive index | 1.410 |
| Acidity | 11.0 |
| Magnetic susceptibility | -37.0×10-6cm3/mol |
| Appearance | White deliquescent crystalline solid |
Chemical Properties
It has a white crystalline colorless solid that has consists of the rapidly heating material. Due to the low melting point it could be decomposed slowly in the air and heated rapidly. The taste like a bitter and burning sensations. Mostly it involves the research and study the organisms in the chemistry lab.
Uses
Potassium cyanide is used in gold mining. It is used in warehouses. Potassium cyanide is used in electroplating process. It is a reagent in analytical chemistry. It is widely used in preparation of carboxylic acid and nitriles. Potassium cyanide is used in the extraction of silver and gold from ores.