Potassium Chromate
Potassium chromate is an inorganic compound that has been treated with the potassium dichromate with the potassium hydroxide. It is obtained from the natural mineral which is known as tarapacaite. These mineral has get from the Atacama desert and in the few cities or areas. In the laboratory it is commonly used chemical for various reactions. The systematic IUPAC name is known as potassium chromate . The chemical or molecular formula of potassium chromate is K2CrO4. The other names are chromic acid and dipotassium salt.
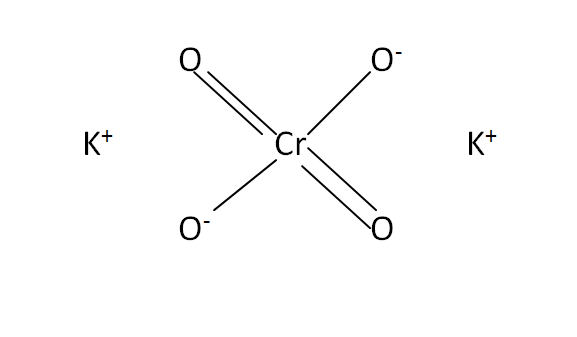
Structural Formula
This is the structural formula of the potassium chromate:
Chemical Formula
The chemical formula of the Potassium chromate is K2CrO4.
Preparation Method
The potassium chromate is prepared by the two ways. One is when the potassium dichromate is reacted with the potassium hydroxide and it gives the formation of potassium chromate. In this reaction the water is evolved. In another method the chromium trioxide is fused with the potassium hydroxide to form the potassium chromate. The following two reactions are given as follows.
K2Cr2O7 + 2KOH → 2K2CrO4 + H2O
2KOH + CrO3 → 2K2CrO4 + H2O
Physical Properties
| Melting point | 968C |
| Boiling point | 1000C |
| Molecular weight | 194.1986g/mol |
| Density | 2.7320g/cm3 |
| Solubility in water | Soluble |
| Refractive index | 1.74 |
| Crystal structure | Rhombic |
| Magnetic susceptibility | -3.9×10-6cm3/mol |
| Appearance | Yellow powder |
| Solubility | Insoluble in alcohol |
Chemical Properties
Potassium chromate is a less hazard to the environment. The melting point is low and the boiling point is very high. It is insoluble in alcohol. The range of magnetic susceptibility is low compared to the other compounds. The crystal structure of potassium chromate is rhombic. It has equal number of molar mass and yellow powder in the appearance.
Uses
Potassium chromate is oxidizing agent in organic process. It is mainly production for making of dyes and also fungicide. It is used for leaching process. It is widely manufacturing the dyeing for textile industry.