Potassium Bromate
Potassium bromate is an iconic compound and a strong oxidizing agent. It is used to making the bread items. According the food corporation reports the potassium bromate is contained 84% in the bread items. It looks like a white crystalline powder. It is used limited and very harmful to us. This is one of the finest inorganic compound also. The systematic IUPAC name is known as potassium bromate. The chemical or molecular formula of potassium bromate is KBrO3. The other names are potassium salt and bromic acid.
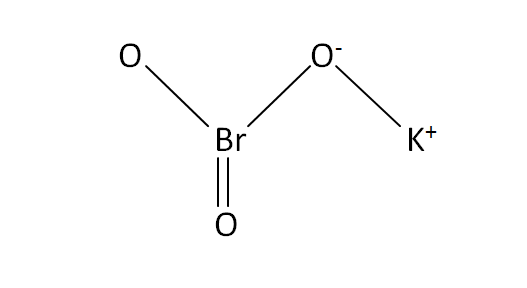
Structural Formula
This is the structural formula of the potassium bromate:
Chemical Formula
The chemical formula of the potassium bromate is KBrO3.
Preparation Method
The preparation of potassium bromate is two ways. One is when the bromine gas is reacted with the potassium hydroxide it produced the potassium hypobromite. These hypobromite is further reacted to the disproportion to get the potassium bromate. The potassium bromide and water is the byproducts. The chemical is given below as follows.
3Br2 + 6KOH → KBrO3 + 5KBr + 3H2O
Physical Properties
| Melting point | 350C |
| Boiling point | 370C |
| Molecular weight | 167.00g/mol |
| Density | 3.27g/cm3 |
| Solubility in water | 49.7g/100mL(100C) |
| Solubility | Insoluble in acetone |
| Crystal structure | Hexagonal |
| Magnetic susceptibility | -52.6×10-6cm3/mol |
| Appearance | White crystalline powder |
Chemical Properties
When the temperature is increased the solubility in water is also increased. Due to the high melting point it will decomposes at the higher temperature. It is one of the non-inflammable substances. The range of ph level is 5-9 at 25C. it is used to manufacturing the lethal substances. This could be the carcinogenic products.
Uses
Potassium bromate is a source of bromine. It is used to prepare the standard solutions that it is stable indefinitely. It is used as an astringent and antiseptic such as toothpaste, mouthwashes and gargles 3 to 5 percent. It is an oxidizing agent for analytic chemistry and also a brominating agent.