Potassium Acetate
Potassium acetate has an equal number of acetate and potassium ions present. It is derived from the potassium salt of acetic acid. In earlier it is used as a expectorants and diuretics. It has associated with the various functions such as physiological and maintaining normal renal function and blood pressure. It has consists of one acetate anion and one potassium cation. These two ions are bonded by the ionic bonds. The systematic IUPAC name is known as potassium acetate. The chemical or molecular formula of potassium acetate is CH3CO2K . It is also known as the potassium ethanoate.
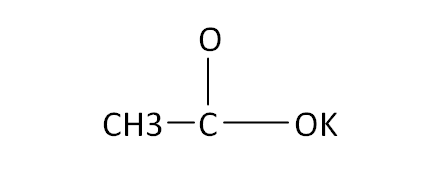
Structural Formula
This is the structural formula of the potassium acetate:
Chemical Formula
The chemical formula of the potassium acetate is CH3CO2K.
Preparation Method
When the potassium hydroxide is reacted with the acetate and it is formed the potassium acetate by the neutralization process. The reaction is as follows.
CH3COOH + KOH → CH3COOK + H2O
By crystallization and evaporation process a small volume of water is added to the potassium carbonate in the acetic solution. It is evolved the hydrogen and oxygen gas.
K2CO3 + 2CH3COOH → 2CH3COOK + H2O + CO2
Physical Properties
| Melting point | 292C |
| Boiling point | Decomposes |
| Molecular weight | 98.142g/mol |
| Density | 1.8g/cm3(20C)
1.57 g/cm3 (25C) |
| Solubility in water | Soluble |
| Acidity | 4.76 |
| Crystal structure | Monoclinic |
| Appearance | White deliquescent crystalline powder |
Chemical Properties
Potassium acetate is a deliquescent crystalline white powder and insoluble in organic solvents like ether, but insoluble in alcohol, ammonia and water. It is odourless and has a faint acetic smell. It is a canonicalised compound. The potassium acetate has a five heavy atoms and zero formal charges. It is used for manufacturing the crystal glasses and medicines.
Uses
Potassium acetate is used in agricultural field. It is mainly used in agricultural chemicals. It is used in the treatment of diabetic ketoacidosis. Potassium acetate is widely used in lubricant making. Usually it is used in fire suppression. It is also a food preservative and it is used in laboratory chemicals. Potassium acetate is used as a catalyst produces of polyurethanes.