Magnesium Phosphate
Magnesium phosphate is an ionic compound that has consists of the combination of magnesium as the cation and phosphate as the anion. It is naturally found in the hydrated water. it can be crystallized through the amorphous forms. The magnesium phosphate consists the electrostatic force of attraction between the magnesium and phosphate. It appears as the white crystalline powder. The systematic IUPAC name is known as magnesium phosphate. The chemical or molecular formula of magnesium phosphate is Mg3(PO4)2.
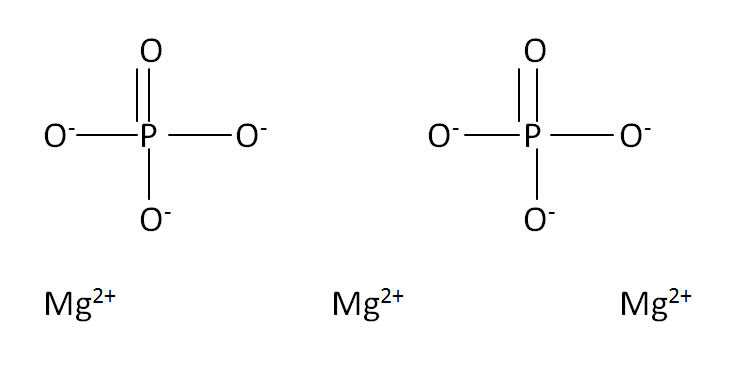
Structural Formula
This is the structural formula of the magnesium phosphate is:
Chemical Formula
The chemical formula of the magnesium phosphate is Mg3(PO4)2.
Preparation Method
When the tribasic orthophosphoric acid is reacted with the magnesium hydroxide in the laboratory by the process of neutralization. It gives the trimagnesium phosphate as the product and water as the byproduct.
2H3PO4+ 3Mg(OH)2 → Mg3(PO4)2 +6H2O
When this product is reacted with the hydrochloric acid it produces magnesium chloride salt and orthophosphoric acid. In the aqueous solution it can be prepared the dimagnesium phosphate dehydrate with the diacidic base is added.
Physical Properties
| Melting point | 1457K |
| Boiling point | 298K |
| Molecular weight | 262.855g/mol |
| Density | 2.195g/cm3 |
| Solubility in water | Soluble |
| Refractive index | 1.7843 |
| Crystal structure | Crystalline |
| Magnetic susceptibility | -15.7×10-6cm3/mol |
| Appearance | White crystalline powder |
| Solubility | Soluble in methanol, ethanol, ether and acetone. |
| Odour | odourless |
Chemical Properties
Magnesium phosphate is a non inflammable substances. It is slightly soluble in methanol, ethanol and acetone. It is odourless chemical compound. It appears as the white crystalline powder. Both the melting point and boiling point is very low. It is soluble in the water and hydrochloric acid.
Uses
Magnesium phosphate is the important essentials for our human body. It supplies the nutrients to all the organs. In the food industry it used as the stabilizer in the food additive. It prevents the deficiency of the vitamin E.