Magnesium Nitrate
Magnesium nitrate is an inorganic compound forms the hydrates when it is present in the air. It is in the hygrocopic solid at the anhydrous form. It occurs naturally abundant in the nature. Being highly soluble in the water. the electrons are freely shared their energy to the other chemical compounds. The magnesium acts as the cations and the nitrate acts as the anions.The systematic IUPAC name is known as magnesium nitrate . The chemical or molecular formula of magnesium nitrate is Mg(NO3)2. It is also known as magnesium dinitrate.
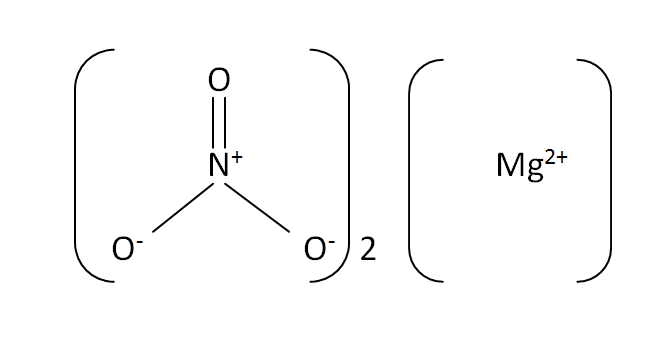
Structural formula
This is the structural formula of the magnesium nitrate:
Chemical Formula
The chemical formula of the magnesium nitrate is Mg(NO3)2.
Preparation Method
When the magnesium hydroxide is reacted with the sodium nitrate it forms the magnesium nitrate is the product and sodium hydroxide is the byproduct. It is a strong acid and it absorbs the nitrogen oxides in the present of air. It can be synthesized only by the use of nitric acid. At the deliquescent water powder it looks like a white crystalline solid. The chemical reaction is given as below.
Mg(NO3)2 + 2NaOH → Mg(OH)2 + 2NaNO3
Physical Properties
| Melting point | 129C |
| Boiling point | 330C |
| Molecular weight | 184.35g/mol |
| Density | 2.0256g/cm3 |
| Solubility in water | 71g/100mL(25C) |
| Refractive index | 1.34 |
| Crystal structure | Cubic |
| Magnetic susceptibility | -23.1×10-6cm3/mol |
| Appearance | White crystalline solid |
| Solubility | Moderately soluble in ethanol and ammonia. |
Chemical Properties
Magnesium nitrate is a non inflammable substances. if the solubility of water is increased, the temperature will be also increased. It is moderately soluble in ethanol and ammonia. The reaction is taken not easily and stable in nature. It looks like a white crystalline solid in the appearance. It is cubic in structure.
Uses
Magnesium nitrate is manufacturing the fertilizers for the plant growth. It controls the pesticides when it sprays on the plant. It acts as a dehydrating agent for preparing the concentrated nitric acid. Naturally it is found in the caverns and mines.