Magnesium Hydroxide
Magnesium hydroxide is an inorganic compound that is used to treat for the constipation problems especially for the childrens. It is naturally occurring more minerals and retain the power. It is also known as the milk of magnesia. It also reduces the stomach acid and increased the bowel movements in the intestines. The systematic IUPAC name is known as magnesium hydroxide. The chemical or molecular formula of acetamide is Mg(OH)2. It is also known as milk of magnesia.
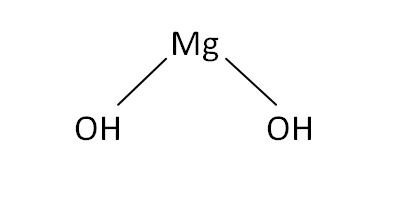
Structural Formula
This is the structural formula of the magnesium hydroxide:
Chemical Formula
The chemical formula of the magnesium hydroxide is Mg(OH)2.
Preparation Method
When the magnesium salts is reacted with the alkaline water which is capable to produce the solid hydroxide by the precipitation process. You can able to produce the more number of tons has been produced.
Mg2+ + 2OH– → Mg(OH)2
There is an another method that is collect the seawater which is the second most abundant mineral present in the seawater is magnesium. Because it contains the more cations presented. In the Industrial scale it is treated with the lime that can be easily produce the magnesium hydroxide. It is more soluble in the fresh water. Whenever you treated with this it dissolves the other minerals from the hydroxide and added to the magnesium. This can be result given is magnesium hydroxide.
Mg2+ + Ca(OH)2 Mg(OH)2 + Ca2+
Physical Properties
| Melting point | 350C |
| Boiling point | decomposes |
| Molecular weight | 58.3197g/mol |
| Density | 2.3446g/cm3 |
| Solubility in water | 0.00064mg/L |
| Refractive index | 1.559 |
| Crystal structure | hexagonal |
| Magnetic susceptibility | -22.1×10-6cm3/mol |
| Appearance | White solid |
Chemical Properties
It is more hazardous and non inflammable. It can be treated as the antacids which is combined in the form of hydrochloric acid and to produce the waters in the stomach. It is approximately 0.5 – 1.5 g works in adults.
Uses
Magnesium hydroxide is used as a suspensions as laxative or antacid. It is widely used as food additive. It mostly used in waste water treatment. Magnesium hydroxide is mainly used in fire retardant and also used in mining of gold. It is usually used in warehouses. Magnesium hydroxide as an excellent in thermal conductor. Magnesium hydroxide is poor in electrical conductor.