Magnesium Chloride
Magnesium chloride is the chemical compound and contains the typical iconic halides that can be highly soluble in water. It can be obtained from the brine or sea water. It is the most abundant mineral and highly content the ancient seabeds. These magnesium metal contributes the 25.5% elemental. The anhydrous is the most priority metal to produce the magnesium metal. The systematic IUPAC name is known as magnesium chloride. The chemical or molecular formula of magnesium chloride is MgCl2. It is also known as the magnesium dichloride.
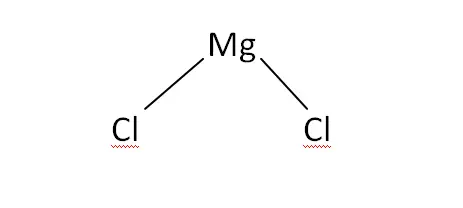
Structural Formula
This is the structural formula of the magnesium chloride:
Chemical Formula
The chemical formula of the magnesium chloride is MgCl2.
Preparation Method
By using the dow process the magnesium chloride is produced by treating the solution of magnesium hydroxide and hydrochloric acid.
Mg(OH)2 + 2HCl → MgCl2 + 2H2O
It crystallizes the cadmium chloride at the high temperatures upto 300oC. by heating the chloride salt it can produced the anhydrous with the hexamine complex to coordinate with the six water ligands. It is required to the absence of the metal by the direct electrolysis with the magnesium metal.
Physical Properties
| Melting point | 714C |
| Boiling point | 1412C |
| Molecular weight | 95.211g/mol |
| Density | 2.32g/cm3 |
| Solubility in water | 7.26mg/L |
| Refractive index | 1.675 |
| Crystal structure | CdCl2 |
| Magnetic susceptibility | -47.4×10-6cm3/mol |
| Appearance | White or colourless crystalline solid |
Chemical Properties
It has contains the high thermal power to heat the solution attains the maximum energy. It has the non inflammable substance. It is capable of an accepting electron pair from the other minerals because it has lewis base. It produces the performance of all the minerals with the effective and contributes to the large industrical scales.
Uses
Magnesium chloride used as magnesium metals. It is mostly in fire extinguishers and food additive. It is flocculating agent. It is also a catalyst. It is widely used in paper manufacturing. Magnesium chloride is used in stabilization, dust control and wind erosion. It is used as lubrication of thread.