Magnesium Carbonate
Magnesium carbonate is a white inorganic salt and that is a colourless to the form of magnesium as exist in the minerals. It has a several hydrate with the possess of the soluble in the water. it consists of the triclinic structure. It is the dehydrate in the form of six oxygen atoms. The systematic IUPAC name is known as magnesium carbonate. The chemical or molecular formula of magnesium carbonate is MgCO3.
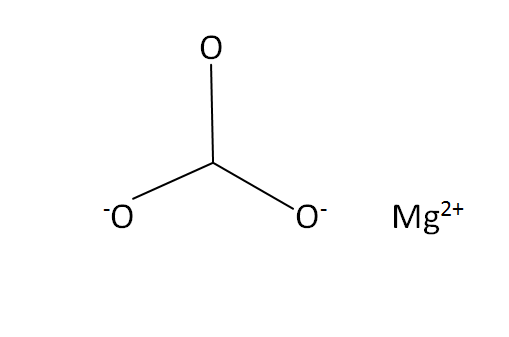
Structural Formula
This is the structural formula of the magnesium carbonate:
Chemical Formula
The chemical formula of the magnesium carbonate is MgCO3.
Preparation Method
When the magnesium chloride and sodium carbonate is reacted and it is produced the magnesium carbonate with the release of sodium chloride and carbon dioxide.
MgCl2 + 2NaHCO3 → MgCO3+2NaCl+H2O+CO2
This is the bicarbonate of slurry of magnesium and the pressure at high temperature. It is reaction between the magnesium bicarbonate and vaccum dried and the industrial scales.
Physical Properties
| Melting point | 990C |
| Boiling point | 333.6C |
| Molecular weight | 84.3139g/mol |
| Density | 2.958g/cm3 |
| Solubility in water | 0.0063mg/L |
| Refractive index | 1.717 |
| Crystal structure | Trigonal |
| Magnetic susceptibility | -32.4×10-6cm3/mol |
| Appearance | Colourless crystalls |
Chemical Properties
With the acids the magnesium carbonate is reacted with the hydrochloric acid and produced the magnesium chloride. At calcination process it is generally decomposed to the liberation of carbon dioxide. During decomposition the lose of water is hydrated. It is important to the production of magnesium carbonate.
Uses
Magnesium carbonate is used as a filtering agent. It is also used for printing of inks. It is mainly used in fire extinguishing. In another way it is used as an anti caking agent of food. Magnesium carbonate is used for making of mineral water and also used in manufacturing of cosmetics. It is used in medical field making of pharmaceutical products.