Magnesium Bromide
Magnesium bromide is the organic compound that is used to sedative the disorders and solve the nerve problems. It is solubility in water and and contains the minerals such as magnesium and bromine. It could be the white and deliquescent. The systematic IUPAC name is known as magnesium bromide. The chemical or molecular formula of magnesium bromide is MgBr2.
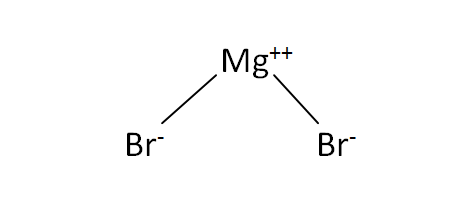
Structural Formula
This is the structural formula of the magnesium bromide:
Chemical Formula
The chemical formula of the magnesium bromide is MgBr2.
Preparation Method
The magnesium bromide is produced by treating with the hydrobromic acid with magnesium oxide. It is used as a solvent free and the combination of calcium channel blockers. it is treated to the hydrobromic acid and leftover in the anhydrous form in the evaporation.
Physical Properties
| Melting point | 711C |
| Boiling point | 1250C |
| Molecular weight | 184.113g/mol |
| Density | 3.72g/cm3 |
| Solubility in water | 102g/100mL |
| Crystal structure | Rhombohedral |
| Magnetic susceptibility | -72.0×10-6cm3/mol |
| Appearance | White hygroscopic hexagonal crysals |
Chemical Properties
It has high melting point and the boiling point and solubility in water. It is hexagonal crystals in the appearance. It has more density and crystal structure and magnetic susceptibility with the molecular weight.
Uses
Magnesium bromide is used as a tranquilizer and it is used as a catalyst. It analyse the regiospecific of triglycerols. It is widely used in the treatment of nervous disorders.usually used as an anticonvulsant. Magnesium bromide is used a mild sedative.