Magnesium Acetate
Magnesium acetate is the organic compound and that contains the two form one is hydrous and another one is anhydrous. In the hydrated form it has a zero health hazards and possessed the more powerful. It is the main source of biological reasons. The systematic IUPAC name is known as magnesium acetate. The chemical or molecular formula of magnesium acetate is Mg(CH3COO)2.
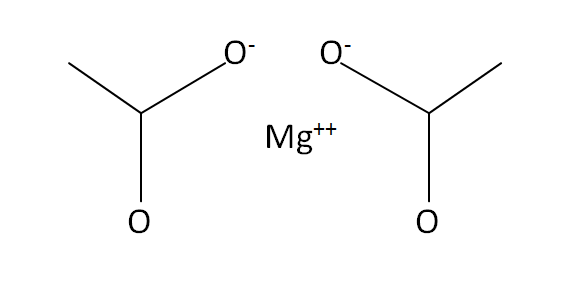
Structural Formula
This is the structural formula of the magnesium acetate:
Chemical Formula
The chemical formula of the magnesium acetate is Mg(CH3COO)2.
Preparation method
The magnesium acetate is prepared by the following three methods.
When the magnesium hydroxide is reated with the acetic acid it produces the magnesium acetate.
2CH3COOH + Mg(OH)2 → Mg(CH3COO)2 + 2H2O
When the magnesium carbonate is reacted with the 20% acetic acid and it gives the magnesium acetate.
2CH3COOH + MgCO3 → Mg(CH3COO)2 + CO2 + H2O
If the magnesium metal is reacted with the acetic acid it will produce the magnesium acetate with the release of hydrogen gas.
Mg + 2 CH3COOH → Mg(CH3COO)2 + H2
Physical Properties
| Melting point | 80C |
| Boiling point | decomposes |
| Molecular weight | 142.394g/mol |
| Density | 1.45g/cm3 |
| Solubility in water | soluble |
| Magnetic susceptibility | -116.0×10-6cm3/mol |
| Appearance | White hydroscopic crystals |
Chemical Properties
It has the more anhydrous metal and it dissolves easily with the acetic solution. It has very low melting point and there is no boiling point. When it is solubility in water it dissolves the solutions in the water. It could be the look of white hydroscopic crystals. When it reacts with the strong solutions and it can be easily decomposed at the effective reactions.
Uses
Magnesium acetate is used in dyeing of textiles and tiles. It is used in offset of printing. It is used as a catalyst in polyester film production. It is used in dialysis solution. It is used to convert the Escherichia coli to the primase.