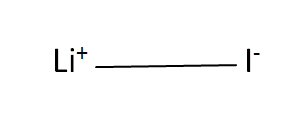Lithium Iodide
Lithium iodide is a chemical compound and it has the oxidation agent. The composition of lithium and iodide is formed the lithium iodide. When we exposed to the air it turns the yellow colour. It can exist in the different forms such as monohydrate, dehydrate and trihydrate. It crystallizes the form of lithium iodide and contains the properties of the chloride motif. The systematic IUPAC name is known as lithium iodide. The chemical or molecular formula of lithium iodide is LiI.
Structural Formula
This is the structural formula of the lithium iodide:
Chemical Formula
The chemical formula of the lithium iodide is LiI.
Preparation Method
When the lithium sulfide is reacted with the strontium iodide and it forms the lithium iodide is the product and strontium sulfide is the byproduct. It goes to the decomposed reaction when it is heated over the threshold limit temperature. It can be synthesized by the various alkali metals and easily combining the particles of the lithium iodide.
Li2S + SeI2 → 2Lil + SrS
Physical Properties
| Melting point | 469C |
| Boiling point | 1171C |
| Molecular weight | 133.85g/mol |
| Density | 4.08g/cm3 |
| Solubility in water | 1510g/100mL(0C) |
| Refractive index | 1.955 |
| Crystal structure | hexagonal |
| Magnetic susceptibility | -50.0×10-6cm3/mol |
| Appearance | White crystalline solid |
| Solubility | Soluble in ethanol, propanol |
Chemical Properties
The boiling point is high and the melting point is low. Lithium iodide is a non inflammable substances. It is hexagonal in the structure. It is soluble in ethanol and propanol. The magnetic susceptibility is very low. It looks like a white crystalline solid in the appearance. It has low density and moderate molar mass.
Uses
Lithium iodide is used for batteries at high temperature as an electrolyte. It is used to cleave C-O bonds. It is made phosphor for neutron detection. In the artificial pacemakers, the lithium batteries is used for long life. It converts methyl esters to carboxylic acids instantly.