Lithium Chloride
Lithium chloride is a chemical compound and it has consists of lithium as positive charged and chloride as the negative charged. It has in the form of anhydrous state. In the polar solvents it acts as the extraordinary chemical compounds. The lithium ion has increase their properties compared to the other alkaline metal chlorides. The systematic IUPAC name is known as lithium chloride . The chemical or molecular formula of lithium chloride is LiCl. It is also known as chlorolithium or lithiumchlorid.
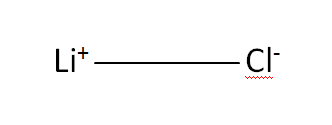
Structural Formula
This is the structural formula of the lithium chloride:
Chemical Formula
The chemical formula of the lithium chloride is LiCl.
Preparation Method
It can be prepared by the reaction between the lithium carbonate with the hydrochloric acid and it gives the lithium chloride as product and hydrogen carbonate as byproduct. In the another way it is highly heated and induced between lithium metal with the anhydrous hydrogen chloride gas or chlorine. Also it is obtained by the steamed the hydrogen chloride by heating on it in the anhydrous form.
Li2CO3 + 2HCl → 2LiCl + H2CO3
Physical Properties
| Melting point | 605C |
| Boiling point | 1382C |
| Molecular weight | 42.394g/mol |
| Density | 2.07g/cm3 |
| Solubility in water | 68.29g/100mL(0C) |
| Refractive index | 1.662 |
| Crystal structure | Octahedral |
| Magnetic susceptibility | -24.3×10-6cm3/mol |
| Appearance | White hygroscopic solid |
| Viscosity | 0.87cP |
Chemical Properties
Lithium chloride is a non inflammable substances. it is more hazardous to the environment. It is the ability to regenerate the anhydrous salt from the hydrates by the process of heating. The boiling point is high and the melting point is low. The viscosity of the lithium bromide is less than 1. It is octahedral in structure. It looks like a white hygroscopic solid in the appearance.
Uses
Lithium chloride is used as an electrolyte in the production of lithium metals. It manufacturing the large dehumidification systems and also used for air conditioning industry. The batteries are prepared by the lithium metal which is light weight for the automobile industries.