Lithium Bromide
Lithium bromide is an ionic compound that has consists the electronic configuration in the ration is 2:1. It is the combination of lithium and bromine. The valence shell of the lithium bromide is one electron. It can exchange the electrons between the lithium and bromine. In the aqueous solution it has contained the deprotonation and dehydration. The solution of lithium bromide is precipitated in this mechanism.The systematic IUPAC name is known as lithium bromide . The chemical or molecular formula of lithium bromide is LiBr.
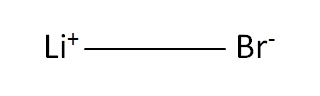
Structural Formula
This is the structural formula of the lithium bromide:
Chemical Formula
The chemical formula of the lithium bromide is LiBr.
Preparation Method
Lithium bromide can be prepared by the many methods. Here we derived the two methods of them. In first method when the lithium carbonate is reacted with the hydrobromic acid it forms the equation of lithium bromide as the product and hydrogen carbonate as the byproduct. In the another method the lithium hydroxide is reacted with the hydrobromic acid or hydrogen bromide it gives the product lithium bromide and water as the byproduct. The chemical reaction is given as follows.
Li2CO3 + 2HBr → 2LiBr +H2CO3
LiOH + HBr → LiBr + H2O
Physical Properties
| Melting point | 552C |
| Boiling point | 1265C |
| Molecular weight | 86.845g/mol |
| Density | 3.464g/cm3 |
| Solubility in water | 143g/100mL(0C) |
| Refractive index | 1.7843 |
| Crystal structure | Cubic |
| Magnetic susceptibility | -34.3×10-6cm3/mol |
| Appearance | White hygroscopic solid |
| Solubility | Soluble in methanol, ethanol, ether and acetone. |
Chemical Properties
Lithium bromide is a non inflammable substances. if the solubility of water is increased, the temperature will be also increased. It is slightly soluble in pyridine. The reaction is taken not easily and stable in nature. It looks like a white hygroscopic solid in the appearance.
Uses
Lithium bromide is used to make air cooling systems work. It is a drying agent for pharmaceuticals industrial purposes. It is widely used for sedative and also in the treatment of epilepsy. It is synthesized the olefins for catalytic dehydrohalogenation.