Hydrogen Sulfate
Hydrogen sulfate is a chemical compound that has the composition of the hydrogen and sulfate. It is a strong acidic agent. It contains one atom of hydrogen and sulfur with the four atoms of oxygen. The ion carries a charge of hydrogen atoms. It is acidic in nature. It is also used for alternative to the sulfuric acid. The systematic IUPAC name is known as hydrogen sulfate. The chemical or molecular name of hydrogen sulfate is HSO4–. It is also known as bisulfate.
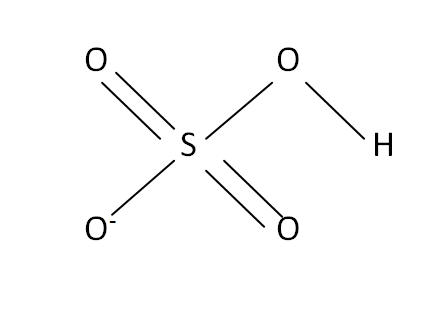
Structural Formula
This is the structural formula of the hydrogen sulfate:
Molecular Formula
The chemical formula of the hydrogen sulfate in HSO4. It has consists of one hydrogen and four oxygen ions compound.
How Can be Prepared
Based on the bronsted lowry it is occurred by the transfer of one proton from one ion or from one molecule to one another. In that way the water acts as the base and reacted with the hydrogen sulfate and gain the proton donor. By the amphoetic nature of the water it acts as the base and acid. It also converts to the hydronium ions. It is chemically equivalent bond.
Physical Properties
| Melting point | 59C |
| Boiling point | 3600C |
| Molecular weight | 97.064g/mol |
| Density | 1826.7Kg/m3 |
| Solubility in water | Soluble |
| Refractive index | 1.45 |
| Complexity | 76 |
| Appearance | White powder |
| Reactive with | Water, nitric acid, ethanol, acetone. |
Chemical Properties
The melting point is low and the boiling point is decomposed. Hydrogen sulfate is a non inflammable substances. It is soluble in water, nitric acid, ethanol and acetone. The complexity is 75. It looks like a white powder in the appearance. It has high density and low molar mass.
Uses
Hydrogen sulfate is used to substitute sulphur-di oxide in the removal of waste water treatment. It is mainly used for soothe skin and reddish blood.