Calcium Iodide
Calcium iodide is the ionic compound which it crystallizes in cubes and it is easily soluble in water. With the use of moisture air decomposes and turns the yellow colour with the liberation of iodine. It is also soluble in ethanol. It is the combination of the calcium and iodine. It appears a white crystals and used as source of salt for cat food. The systematic IUPAC name is known as calcium iodide. The chemical or molecular name of calcium iodide is CaI2.
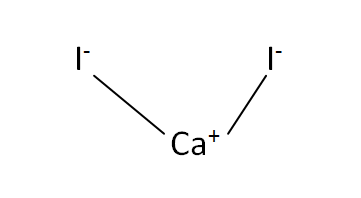
Structural Formula
This is the structural formula of the calcium iodide:
Chemical Formula
The chemical formula of the calcium iodide is CaI2. It has consists of calcium ions and iodide ions compounds.
How it can Be Prepared
The calcium iodide can be prepared by reacting calcium carbonate with the hydroxidic acid or hydrogen iodide gas. It can be easily soluble in the water. this salt has a highly deliquescent solid in the appearance. When it is slowly reacts with the oxygen or carbon dioxide it could be liberated the iodine which is faint yellow colour. The chemical reaction is given as follows.
CaCO3 + 2 HI → CaI2 + H2O + CO2
Physical Properties
| Melting point | 779C |
| Boiling point | 1100C |
| Molecular weight | 293.887g/mol |
| Density | 3.956g/cm3 |
| Solubility in water | 64.6g/100mL |
| Refractive index | 1.49 |
| Crystal structure | Rhombohedral |
| Magnetic susceptibility | -109.0×10-6cm3/mol |
| Appearance | White solid |
| Solubility | Soluble in acetones and alcohols |
Chemical Properties
Calcium iodide is a white solid or crystals. It is a strongly base chemical compounds. But it reacts with the both acid and basic forms. It has low density and high molecular weight. There is low melting point and high boiling point. It has bitter in taste.
Uses
Calcium iodide is mainly used for medicines and also a photography. calcium iodide is used as a iodine source of cat food. It increases the strong of tooth enamel at the time of filling it.