Calcium Chloride
Calcium chloride is the chemical inorganic compound and contains the typical iconic halides that can be highly soluble in water. It can be obtained from the brine. It is the most abundant mineral and highly content the ancient seabeds. These calcium metal contributes the 25.5% elemental. The anhydrous is the most priority metal to produce the calcium chloride. The systematic IUPAC name is known as calcium chloride. The chemical or molecular name of calcium chloride is CaCl2.
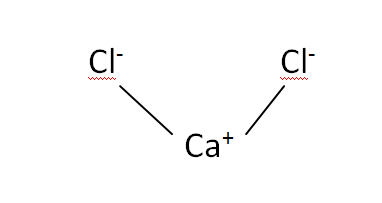
Structural Formula
This is the structural formula of the calcium chloride:
Chemical Formula
The chemical formula of the calcium chloride is CaCl2. It has consists of one calcium and two chloride ions.
Reactions
By using the solvay process the calcium chloride is produced by treating the solution of sodium chloride and calcium carbonate. It crystallizes the calcium chloride at the high temperatures upto 1935oC. By heating the chloride salt it can produced the anhydrous with the hexamine complex to coordinate with the six water ligands. It is obtained from the purifaction of brine at room temperatures. These can also electrolysed and give the calcium with the chlorine gas.
2 NaCl + CaCO3 → CaCl2 + Na2CO3
Physical Properties
| Melting point | 772C |
| Boiling point | 1935C |
| Molecular weight | 110.98g/mol |
| Density | 2.15g/cm3 |
| Solubility in water | 59.5g/100mL |
| Refractive index | 1.52 |
| Crystal structure | Orthorhombic |
| Magnetic susceptibility | -5.47×10-6cm3/mol |
| Appearance | White hygroscopic powder |
| Solubility | Soluble in acetic acids, alcohols. Insoluble in liquid ammonia. |
| Odor | odorless |
Chemical Properties
Calcium chloride is a less hazard to the environment. The melting point is low and the boiling point is very high. It is insoluble in liquid ammonia. The range of magnetic susceptibility is low compared to the other compounds. The crystal structure of calcium chloride is orthorhombic. It has equal number of molar mass and white powder in the appearance.
Uses
Calcium chloride is used as a sterilant for male animals. It is mainly used for heating pads and self heating cans. It is used as an electrolyte for sports drinks and also production of charcoal.