Calcium Bromide
Calcium bromide is a chemical compound that has consists of calcium and bromide ions. It is a colourless crystalline solid and has a strong oxidizing smell agent. It is acidic in nature and acts as basic. It is soluble in water and non combustible substances. It is mainly used in the some drilling fluids in the hydrated form. The systematic IUPAC name is known as calcium bromide. The chemical or molecular name of calcium bromide is CaBr2. It is also known as calcium dibromide.
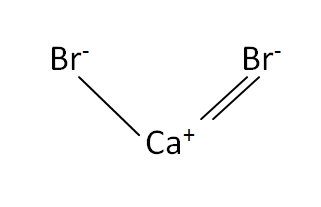
Structural Formula
This is the structural formula of the calcium bromide:
Chemical Formula
The chemical formula of the calcium bromide is CaBr2. It has consists of one calcium and two bromide ions.
How the Calcium Bromide is Produced
When the calcium oxide is reacts with the bromine that has been strongly heated in the air and it gives the results of calcium bromide as the product and oxygen as the byproduct. It also crystallizes in an aqueous solution to exposed in the carbon dioxide rich atmosphere. The oxygen help out the bromide to bromine. The chemical reaction is given as follows.
2 CaO + 2Br2 → 2CaBr2 + O2
Physical Properties
| Melting point | 730C |
| Boiling point | 1815C |
| Molecular weight | 199.89g/mol |
| Density | 3.353g/cm3 |
| Solubility in water | 125g/100mL |
| Refractive index | 1.34 |
| Crystal structure | Rhomboid |
| Magnetic susceptibility | -73.8×10-6cm3/mol |
| Appearance | Colourless crystals |
| Solubility | Soluble in alcohol and acetone. |
Chemical Properties
The boiling point is high and the melting point is low. Calcium bromide is a non combustible substances. It is monoclinic in the structure. It is soluble in alcohol and acetone. The magnetic susceptibility is low. It looks like a colourless crystals in the appearance. It has low density and moderate molar mass.
Uses
Calcium bromide is used for wood preservative. It is mainly used for photography. It is component used for high density clear drilling.