Cadmium Sulfate
Cadmium sulfate is an inorganic compound with the combination of the cadmium and sulfate. It has a water soluble solid. cadmium sulfate is a natural resources that has found abundant naturally. It is insoluble in ethanol and odorless solid. These minerals can be separated from the other minerals like kainite which is the primary sources of the cadmium chemicals. The systematic IUPAC name is known as cadmium sulfate. The chemical or molecular name of cadmium sulfate is CdSO4. It is also known as sulfuric acid or cadmium salt.
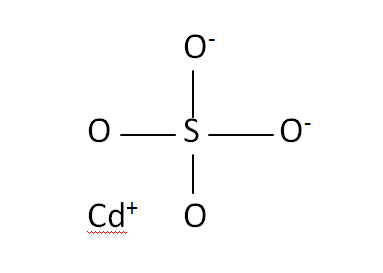
Structural Formula
This is the structural formula of the cadmium sulfate:
Chemical Formula
The chemical formula of the cadmium sulfate is CdSO4. It has consists of sulfate and cadmium ions.
How it is Prepared
The cadmium sulfate can be prepared by the process of exothermic reactions. In this process , cadmium oxide is reacted with the dilute sulfuric acid that is formed cadmium sulfate as the product and water as the byproduct. Then it has been dissolved in hot water, filtered and evaporated to a cuticle. It can also prepared by the endothermic process, when the cadmium metal is reacted with the dilute sulfuric acid and it gives the result of cadmium sulfate.
CdO + H2SO4 → CdSO4 + H2O
Cd + H2SO4 → CdSO4 + H2
Physical Properties
| Melting point | 1000C |
| Boiling point | Decomposes |
| Molecular weight | 208.47g/mol |
| Density | 4.691g/cm3 |
| Solubility in water | 75g/100mL |
| Refractive index | 1.565 |
| Crystal structure | Orthorhombic |
| Magnetic susceptibility | -59.2×10-6cm3/mol |
| Appearance | White hygroscopic solid |
| Solubility | Slightly soluble in methanol and ethyl acetate. Insoluble in ethanol. |
| Odor | odourless |
Chemical Properties
Cadmium sulfate is a non combustible substances. It is dangerous to the environment. It is highly soluble in the water. In the thermochemistry it has standary enthalpy and molar entropy. It produced the common form known as monohydrate.
Uses
Cadmium sulfate is used for pigments and electroplating. It is mainly used for electrode position such as fungicides, bactericides and lubricants. It is widely used as an accelerator for cementry formation.