Barium Sulfate
Barium sulfate is an inorganic compound that has consists of barium and sulfate. The barium has positively charged and sulfate has negatively charged and acts as the cations and anions respectively. It requires to obtain the coke and then seggrated from the impurities. It occurs naturally in the mineral barite. After the process of mining it has been obtained from the barite. The systematic IUPAC name is known as barium sulfate. The chemical or molecular name of barium sulfate is BaSO4.
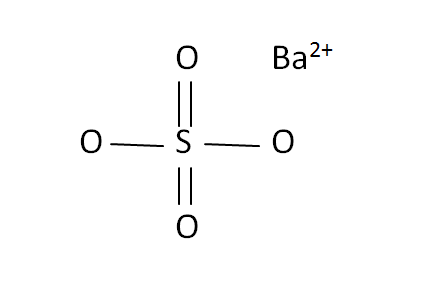
Structural Formula
This is the structural formula of the barium sulfate:
Chemical Formula
The chemical formula of the barium sulfate in BaSO4. It has consists of one barium ions and four oxide ions with sulfate.
What Method the Barium Sulfate Can be Produced
It can be prepared by the reaction between the barium sulfide and sulfuric acid at the room temperatures. The overall equations is given as follows. It has been converted to the oxide, carbonate and halides. The barium ions is purely soluble in the water. It has been impurities from the barite. The chemical reaction is given as follows.
BaS + H2SO4 → BaSO4+ H2S
Physical Properties
| Melting point | 1580C |
| Boiling point | 1600C |
| Molecular weight | 233.38g/mol |
| Density | 4.49g/cm3 |
| Solubility in water | 24.48g/100mL |
| Refractive index | 1.636 |
| Crystal structure | Orthorhombic |
| Magnetic susceptibility | -71.3×10-6cm3/mol |
| Appearance | White crystalline |
| Solubility | Soluble in concentrated hot sulphuric acid. Insoluble in alcohol. |
| Odor | odourless |
Chemical Properties
Barium sulfate is a non combustible substances. It is more hazardous to the environment. It is the ability to regenerate the anhydrous salt from the hydrates by the process of heating. The boiling point is high and the melting point is low. It is orthorhombic in structure. It looks like a white crystalline in the appearance.
Applications
Barium sulfate is used as a radiopaque agent. It is mainly used for concrete shields and oil well drilling fluids. It is used for paper coatings, textile and catalyst supports.