Barium Nitrate
Barium nitrate is an inorganic compound that the combination of the barium salt with the nitric acid. Barium nitrate is a colourless, water soluble and toxic substances. The nitrobarite is the rare mineral in the group of barium compounds. Commonly it has been used in the pyrotechnics. It can be exploded under the right conditions when it is reacted to heat. It is one of the non combustible chemicals.The systematic IUPAC name is known as Barium nitrate. The chemical or molecular formula of Barium nitrate is Ba(NO3)2. It is also known as barium salt or barium dinitrite.
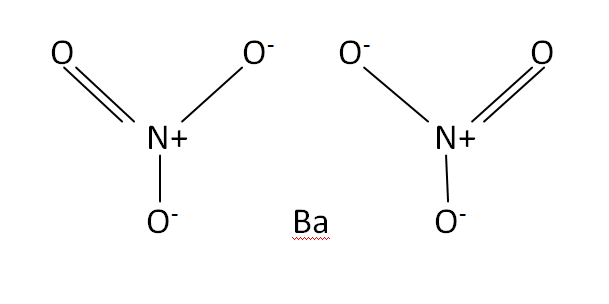
Structural Formula
This is the structural formula of the Barium nitrate:
Chemical Formula
The chemical formula or molecular formula is Ba(NO3)2.
Preparation Method
The barium nitrate can be prepared by the two methods. In the first method the barium carbonate is reacted with the nitric acid and it precipitate iron impurities to the surface. Then by using the filtration techniques this impurities are removed. Then the remaining solution is undergo to the evaporation process. After completed the evaporation it crystallizes to producing the barium nitrate. In the second method the barium sulfide is reacted with the nitric acid to producing the barium nitrate. But in this method the barium nitrate is decomposed and it gives the barium oxide, nitrogen dioxide and oxygen.
2Ba(NO3)2 → 2BaO + 4NO2 + O2
Physical Properties
| Melting point | 592C |
| Boiling point | 83C |
| Molecular weight | 261.337g/mol |
| Density | 3.24g/cm3 |
| Solubility in water | 0.00064mg/L |
| Refractive index | 1.5659 |
| Crystal structure | cubic |
| Magnetic susceptibility | -66.5×10-6cm3/mol |
| Appearance | White lustrous crystals |
| Odor | Odourless |
| Solubility | Soluble in acetone, ethanol |
Chemical Properties
Barium nitrate is a white lustrous crystals that has been obtained abundantly. It is soluble in acetone and ethanol. It is cubic in structure. The melting point is greater than the boiling point. It has moderate molar mass and low density.
Uses
Barium nitrate is used in production of barium oxide. It is also used in making of ceramic glazes. It is an oxidizing agent. It is widely used for detonators. It is mainly used for paints and making explosives.