Barium Iodide
Barium iodide is an inorganic compound that has the combination of barium and iodine. It is an ionic compound because it is able to accept the electronic configuration of the atoms. This has been accepted by the donating the electrons to the ionic substances. Barium iodide is soluble in water and insoluble in acetone and methanol. It could be the change of paking in the anhydrous form. The systematic IUPAC name is known as Barium iodide. The chemical or molecular formula of Barium iodide is Bal2.
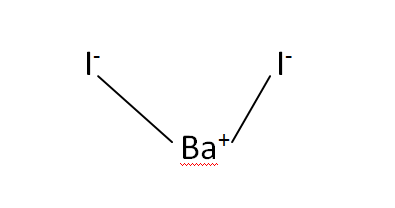
Structural Formula
This is the structural formula of the Barium iodide:
Chemical Formula
The chemical formula of the Barium iodide is Bal2.
Preparation Method
When the barium metal is reacted with the 1,2- diiodoethane in ether it forms the barium iodide. Further it is reacted with the alkyl potassium compounds to form the organobarium compounds. The organobarium is a carbon bonded to bond with the any 2 group of elements. It is widely used in the organic chemistry. If you want the barium metal, then it is highly active with the lithium biphenyl compound to reduced.
Physical Properties
| Melting point | 711C |
| Boiling point | 2027C |
| Molecular weight | 391.136g/mol |
| Density | 5.15g/cm3 |
| Solubility in water | 166.7g/mL(0C) |
| Odor | odorless |
| Crystal structure | Orthorhombic |
| Magnetic susceptibility | -124.1×10-6cm3/mol |
| Appearance | White orthorhombic crystals |
Chemical Properties
Barium iodide is a highly toxic substances. it has a odorless compound and orthorhombic in structure. It looks like a white crystals in the appearance. It has consists of both forms such as anhydrous and hydrated form. The boiling point is very high and the melting point is very low. It has a low density and high molecular weight. The magnetic susceptibility is very high level.
Uses
Barium iodide is used in the preparation of barium dioxide, not for medical use. It is widely used in the manufacture of other iodide compounds. It is used to densify the copper castings.