Barium Hydroxide
Barium hydroxide is a chemical compound that can be made up of one atom of barium, two atoms of hydrogen and two atoms of oxygen. It has no odourless and looks like a white powder naturally. It is obtained through the dissolving in water. The barium hydroxide is so harmful when you inhaled it or swallowed it. It has more toxicity and more corrosiveness. The systematic IUPAC name is known as Barium hydroxide. The chemical or molecular formula of Barium hydroxide is Ba(OH)2. It is also known as barium dihydroxide or caustic baryta.
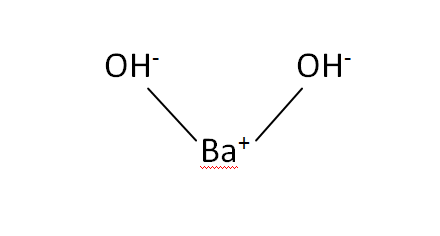
Structural Formula
This is the structural formula of the barium hydroxide:
Chemical Formula
The chemical formula or molecular formula is Ba(OH)2.
Preparation Method
The barium hydroxide can be prepared by the reaction of dissolving barium oxide in the water. The octahydrate is crystallized when it converts to the monohydrate at the heating in air. It has six water hydroxide ligands. These barium oxide is very useful to the manufacturing the chemicals in the industry. The chemical reaction is given as follows.
BaO + H2O → Ba(OH)2
Physical Properties
| Melting point | 78C |
| Boiling point | 780C |
| Molecular weight | 171.34g/mol |
| Density | 3.743g/cm3 |
| Solubility in water | 1.67g/100mL(0C) |
| Refractive index | 1.50 |
| Crystal structure | Octahedral |
| Magnetic susceptibility | -53.2×10-6cm3/mol |
| Appearance | White solid |
| Basicity | 0.15 |
Chemical Properties
It has a mousy odour and it is in the form of octahedral struture. The basicity is accompanied by the barium hydroxide. It looks lika a white solid in the appearance. The melting and boiling point is very low. It has a low density and moderate molecular mass.
Uses
Barium hydroxide use of barium hydroxide rather than soda lime. It is also used in the manufacture of barium soaps,alkalis,grease etc… It is also used to the treatment of sewage water.