Barium Chloride
Barium chloride is an inorganic salt that has the combination of barium and chloride. It is toxic at naturally. The barium acts as the cations and the chloride acts as the anions. Barium chloride is a hygroscopic and blow at the yellow green colour when it is put at the furnace. The systematic IUPAC name is known as Barium chloride. The chemical or molecular formula of Barium chloride is BaCl2.
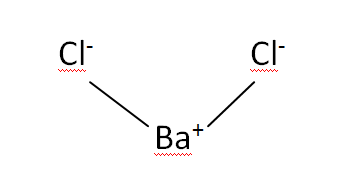
Structural Formula
This is the structural formula of the Barium chloride:
Chemical Formula
The chemical formula or molecular formula is BaCl2.
Preparation Method
Take the concentrated hydrochloric acid 180ml when it is diluted with the 400ml of water. the barium carbonate is mixture with the hydrochloric acid in the vessel. When the solution is neutral to litmus then it will be boiling. This barium carbonate is cosists the iron as an impurity. By adding the 3g of barium peroxide then the solution is heated for half an hour. Then this solution is prevented from the crystallization and it will be cooling. At the room temperature the distilled water is crystallized under the dilute hydrochloric acid.
BaCO3 + 2HCl → BaCl2 + CO2 + H2O
Physical Properties
| Melting point | 962C |
| Boiling point | 1560C |
| Molecular weight | 208.23g/mol |
| Density | 3.856g/cm3 |
| Solubility in water | 31.2g/100mL(0C) |
| Odor | Odourless |
| Crystal structure | Orthogonal |
| Magnetic susceptibility | -72.1×10-6cm3/mol |
| Appearance | White solid |
| Solubility | Soluble in methanol, insoluble in ethanol |
Chemical Properties
When the barium chloride is mixed with the sodium sulfate and it will cause a double substitution reaction. The pH of the solution does not affects and it remains neutral. It is insoluble in ethanol and soluble in methanol. The melting point is low and the boiling point is high.
Uses
Barium chloride is used as raw material to produce salts. It is also used in the manufacture of rubber. It is widely used in hardening of steel. Barium chloride is used in paper making industry. It is used in the manufacturing of pigments. It is used for the purification of heat treatment cells.